Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension
- PMID: 24047466
- PMCID: PMC3931121
- DOI: 10.1165/rcmb.2013-0191OC
Sirtuin 3 deficiency does not augment hypoxia-induced pulmonary hypertension
Abstract
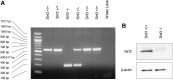
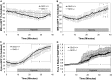
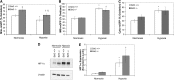
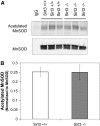
Alveolar hypoxia elicits increases in mitochondrial reactive oxygen species (ROS) signaling in pulmonary arterial (PA) smooth muscle cells (PASMCs), triggering hypoxic pulmonary vasoconstriction. Mice deficient in sirtuin (Sirt) 3, a nicotinamide adenine dinucleotide-dependent mitochondrial deacetylase, demonstrate enhanced left ventricular hypertrophy after aortic banding, whereas cells from these mice reportedly exhibit augmented hypoxia-induced ROS signaling and hypoxia-inducible factor (HIF)-1 activation. We therefore tested whether deletion of Sirt3 would augment hypoxia-induced ROS signaling in PASMCs, thereby exacerbating the development of pulmonary hypertension (PH) and right ventricular hypertrophy. In PASMCs from Sirt3 knockout (Sirt3(-/-)) mice in the C57BL/6 background, we observed that acute hypoxia (1.5% O2; 30 min)-induced changes in ROS signaling, detected using targeted redox-sensitive, ratiometric fluorescent protein sensors (roGFP) in the mitochondrial matrix, intermembrane space, and the cytosol, were indistinguishable from Sirt3(+/+) cells. Acute hypoxia-induced cytosolic calcium signaling in Sirt3(-/-) PASMCs was also indistinguishable from Sirt3(+/+) cells. During sustained hypoxia (1.5% O2; 16 h), Sirt3 deletion augmented mitochondrial matrix oxidant stress, but this did not correspond to an augmentation of intermembrane space or cytosolic oxidant signaling. Sirt3 deletion did not affect HIF-1α stabilization under normoxia, nor did it augment HIF-1α stabilization during sustained hypoxia (1.5% O2; 4 h). Sirt3(-/-) mice housed in chronic hypoxia (10% O2; 30 d) developed PH, PA wall remodeling, and right ventricular hypertrophy that was indistinguishable from Sirt3(+/+) littermates. Thus, Sirt3 deletion does not augment hypoxia-induced ROS signaling or its consequences in the cytosol of PASMCs, or the development of PH. These findings suggest that Sirt3 responses may be cell type specific, or restricted to certain genetic backgrounds.
Figures

References
-
- Waypa GB, Guzy R, Mungai PT, Mack MM, Marks JD, Roe MW, Schumacker PT. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. - PubMed
-
- Waypa GB, Marks JD, Mack MM, Boriboun C, Mungai PT, Schumacker PT. Mitochondrial reactive oxygen species trigger calcium increases during hypoxia in pulmonary arterial myocytes. Circ Res. 2002;91:719–726. - PubMed
-
- Waypa GB, Chandel NS, Schumacker PT. Model for hypoxic pulmonary vasoconstriction involving mitochondrial oxygen sensing. Circ Res. 2001;88:1259–1266. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

