Pd(0)-catalyzed 1,1-diarylation of ethylene and allylic carbonates
- PMID: 24047468
- PMCID: PMC3955058
- DOI: 10.1021/ol4023358
Pd(0)-catalyzed 1,1-diarylation of ethylene and allylic carbonates
Abstract
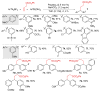
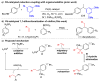
An efficient protocol for the one-step synthesis of biologically relevant 1,1-diarylalkanes has been described. This reaction introduces two different aryl groups across the terminal end of simple feedstock alkenes such as ethylene and allylic carbonates. The propensity to generate π-benzylpalladium intermediates dictates the exclusive 1,1-regioselectivity observed in the product.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Messaoudi S, Hamze A, Provot O, Treguier B, Rodrigo DLJ, Bignon J, Liu JM, Wdzieczak-Bakala J, Thoret S, Dubois J, Brion JD, Alami M. Chem Med Chem. 2011;6:488. - PubMed
- Moree WJ, Li BF, Zamani-Kord S, Yu J, Coon T, Huang C, Marinkovic D, Tucci FC, Malany S, Bradbury MJ, Hernandez LM, Wen J, Wang H, Hoare SRJ, Petroski RE, Jalali K, Yang C, Sacaan A, Madan A, Crowe PD, Beaton G. Bioorg Med Chem Lett. 2010;20:5874. - PubMed
- Cheltsov AV, Aoyagi M, Aleshin A, Yu ECW, Gilliland T, Zhai D, Bobkov AA, Reed JC, Liddington RC, Abagyan R. J Med Chem. 2010;53:3899. - PMC - PubMed
- Hu Q, Yin L, Jagusch C, Hille UE, Hartmann RW. J Med Chem. 2010;53:5049. - PubMed
- Liang H, Wu X, Yalowich JC, Hasinoff BB. Mol Pharmacol. 2008;73:686. - PubMed
- Moriconi A, Cesta MC, Cervellera MN, Aramini A, Coniglio S, Colagioia S, Beccari AR, Bizzarri C, Cavicchia MR, Locati M, Galliera E, Di BP, Vigilante P, Bertini R, Allegretti M. J Med Chem. 2007;50:3984. - PubMed
- Barda DA, Wang ZQ, Britton TC, Henry SS, Jagdmann GE, Coleman DS, Johnson MP, Andis SL, Schoepp DD. Bioorg Med Chem Lett. 2004;14:3099. - PubMed
-
- Gligorich KM, Cummings SA, Sigman MS. J Am Chem Soc. 2007;129:14193. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous