The early fetal development of human neocortical GABAergic interneurons
- PMID: 24047602
- PMCID: PMC4318531
- DOI: 10.1093/cercor/bht254
The early fetal development of human neocortical GABAergic interneurons
Abstract
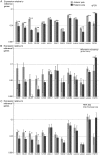
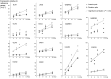
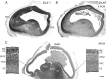
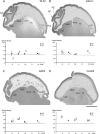
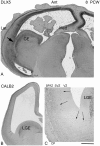
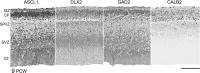
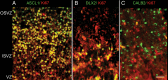
GABAergic interneurons are crucial to controlling the excitability and responsiveness of cortical circuitry. Their developmental origin may differ between rodents and human. We have demonstrated the expression of 12 GABAergic interneuron-associated genes in samples from human neocortex by quantitative rtPCR from 8 to 12 postconceptional weeks (PCW) and shown a significant anterior to posterior expression gradient, confirmed by in situ hybridization or immunohistochemistry for GAD1 and 2, DLX1, 2, and 5, ASCL1, OLIG2, and CALB2. Following cortical plate (CP) formation from 8 to 9 PCW, a proportion of cells were strongly stained for all these markers in the CP and presubplate. ASCL1 and DLX2 maintained high expression in the proliferative zones and showed extensive immunofluorescent double-labeling with the cell division marker Ki-67. CALB2-positive cells increased steadily in the SVZ/VZ from 10 PCW but were not double-labeled with Ki-67. Expression of GABAergic genes was generally higher in the dorsal pallium than in the ganglionic eminences, with lower expression in the intervening ventral pallium. It is widely accepted that the cortical proliferative zones may generate CALB2-positive interneurons from mid-gestation; we now show that the anterior neocortical proliferative layers especially may be a rich source of interneurons in the early neocortex.
Keywords: DLX genes; GABA; GABRB3; cerebral cortex; inhibitory interneurons; neurodevelopmental disorders.
© The Author 2013. Published by Oxford University Press.
Figures


References
-
- Bayatti N, Moss JA, Sun L, Ambrose P, Ward JFH, Lindsay S, Clowry GJ. A molecular neuroanatomical study of the developing human neocortex from 8 to 17 postconceptional weeks revealing the early differentiation of the subplate and subventricular zone. Cereb Cortex. 2008;18:1536–1548. doi: 10.1093/cercor/bhm184. - DOI - PMC - PubMed
-
- Bayatti N, Sarma S, Shaw C, Eyre JA, Vouyiouklis DA, Lindsay S, Clowry GJ. Progressive loss of PAX6, TBR2, NEUROD and TBR1 mRNA gradients correlates with translocation of EMX2 to the cortical plate during human cortical development. Eur J Neurosci. 2008;28:1449–1456. doi: 10.1111/j.1460-9568.2008.06475.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

