The importance of size and disorder in the cryoprotective effects of dehydrins
- PMID: 24047864
- PMCID: PMC3813657
- DOI: 10.1104/pp.113.226803
The importance of size and disorder in the cryoprotective effects of dehydrins
Abstract
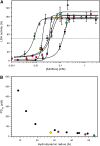
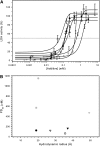
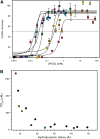
Dehydrins protect plant proteins and membranes from damage during drought and cold. Vitis riparia K2 is a 48-residue protein that can protect lactate dehydrogenase from freeze-thaw damage by preventing the aggregation and denaturation of the enzyme. To further elucidate its mechanism, we used a series of V. riparia K2 concatemers (K4, K6, K8, and K10) and natural dehydrins (V. riparia YSK2, 60 kilodalton peach dehydrin [PCA60], barley dehydrin5 [Dhn5], Thellungiella salsuginea dehydrin2 [TsDHN-2], and Opuntia streptacantha dehydrin1 [OpsDHN-1]) to test the effect of the number of K-segments and dehydrin size on their ability to protect lactate dehydrogenase from freeze-thaw damage. The results show that the larger the hydrodynamic radius of the dehydrin, the more effective the cryoprotection. A similar trend is observed with polyethylene glycol, which would suggest that the protection is simply a nonspecific volume exclusion effect that can be manifested by any protein. However, structured proteins of a similar range of sizes did not show the same pattern and level of cryoprotection. Our results suggest that with respect to enzyme protection, dehydrins function primarily as molecular shields and that their intrinsic disorder is required for them to be an effective cryoprotectant. Lastly, we show that the cryoprotection by a dehydrin is not due to any antifreeze protein-like activity, as has been reported previously.
Figures




References
-
- Allagulova CR, Gimalov FR, Shakirova FM, Vakhitov VA. (2003) The plant dehydrins: structure and putative functions. Biochemistry (Mosc) 68: 945–951 - PubMed
-
- Alsheikh MK, Heyen BJ, Randall SK. (2003) Ion binding properties of the dehydrin ERD14 are dependent upon phosphorylation. J Biol Chem 278: 40882–40889 - PubMed
-
- Anchordoquy TJ, Carpenter JF. (1996) Polymers protect lactate dehydrogenase during freeze-drying by inhibiting dissociation in the frozen state. Arch Biochem Biophys 332: 231–238 - PubMed
-
- Andrews P. (1970) Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal 18: 1–53 - PubMed
-
- Bravo LA, Close TJ, Corcuera LJ, Guy CL. (1999) Characterization of an 80-kDa dehydrin-like protein in barley responsive to cold acclimation. Physiol Plant 106: 177–183
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

