Structure of a PLS-class pentatricopeptide repeat protein provides insights into mechanism of RNA recognition
- PMID: 24047899
- PMCID: PMC3814750
- DOI: 10.1074/jbc.M113.496828
Structure of a PLS-class pentatricopeptide repeat protein provides insights into mechanism of RNA recognition
Abstract
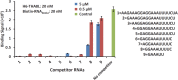
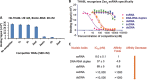
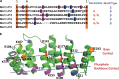
Pentatricopeptide repeat (PPR) proteins are sequence-specific RNA-binding proteins that form a pervasive family of proteins conserved in yeast, plants, and humans. The plant PPR proteins are grouped mainly into the P and PLS classes. Here, we report the crystal structure of a PLS-class PPR protein from Arabidopsis thaliana called THA8L (THA8-like) at 2.0 Å. THA8L resembles THA8 (thylakoid assembly 8), a protein that is required for the splicing of specific group II introns of genes involved in biogenesis of chloroplast thylakoid membranes. The THA8L structure contains three P-type PPR motifs flanked by one L-type motif and one S-type motif. We identified several putative THA8L-binding sites, enriched with purine sequences, in the group II introns. Importantly, THA8L has strong binding preference for single-stranded RNA over single-stranded DNA or double-stranded RNA. Structural analysis revealed that THA8L contains two extensive patches of positively charged residues next to the residues that are proposed to comprise the RNA-binding codes. Mutations in these two positively charged patches greatly reduced THA8L RNA-binding activity. On the basis of these data, we constructed a model of THA8L-RNA binding that is dependent on two forces: one is the interaction between nucleotide bases and specific amino acids in the PPR motifs (codes), and the other is the interaction between the negatively charged RNA backbone and positively charged residues of PPR motifs. Together, these results further our understanding of the mechanism of PPR protein-RNA interactions.
Keywords: Chloroplast; Crystal Structure; Pentatricopeptide Repeat (PPR) Protein; Plant Biochemistry; RNA Processing; RNA-binding Protein; THA8.
Figures



References
-
- Small I. D., Peeters N. (2000) The PPR motif–a TPR-related motif prevalent in plant organellar proteins. Trends Biochem. Sci. 25, 46–47 - PubMed
-
- Lurin C., Andrés C., Aubourg S., Bellaoui M., Bitton F., Bruyère C., Caboche M., Debast C., Gualberto J., Hoffmann B., Lecharny A., Le Ret M., Martin-Magniette M. L., Mireau H., Peeters N., Renou J. P., Szurek B., Taconnat L., Small I. (2004) Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16, 2089–2103 - PMC - PubMed
-
- Fujii S., Small I. (2011) The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 191, 37–47 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

