Tumour heterogeneity and cancer cell plasticity
- PMID: 24048065
- PMCID: PMC4521623
- DOI: 10.1038/nature12624
Tumour heterogeneity and cancer cell plasticity
Abstract
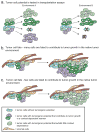
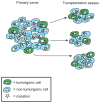
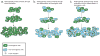
Phenotypic and functional heterogeneity arise among cancer cells within the same tumour as a consequence of genetic change, environmental differences and reversible changes in cell properties. Some cancers also contain a hierarchy in which tumorigenic cancer stem cells differentiate into non-tumorigenic progeny. However, it remains unclear what fraction of cancers follow the stem-cell model and what clinical behaviours the model explains. Studies using lineage tracing and deep sequencing could have implications for the cancer stem-cell model and may help to determine the extent to which it accounts for therapy resistance and disease progression.
Figures


References
-
- Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. - PubMed
-
- Kummermehr J, Trott K-R. In: Stem Cells. Potten CS, editor. Academic Press; 1997. pp. 363–399.
-
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. - PubMed
-
- Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. - PubMed
-
- Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

