Small molecules present in the cerebrospinal fluid metabolome influence superoxide dismutase 1 aggregation
- PMID: 24048249
- PMCID: PMC3794824
- DOI: 10.3390/ijms140919128
Small molecules present in the cerebrospinal fluid metabolome influence superoxide dismutase 1 aggregation
Abstract
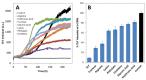
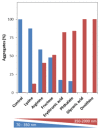
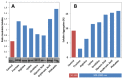
Superoxide dismutase 1 (SOD1) aggregation is one of the pathological markers of amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disorder. The underlying molecular grounds of SOD1 pathologic aggregation remains obscure as mutations alone are not exclusively the cause for the formation of protein inclusions. Thus, other components in the cell environment likely play a key role in triggering SOD1 toxic aggregation in ALS. Recently, it was found that ALS patients present a specific altered metabolomic profile in the cerebrospinal fluid (CSF) where SOD1 is also present and potentially interacts with metabolites. Here we have investigated how some of these small molecules affect apoSOD1 structure and aggregation propensity. Our results show that as co-solvents, the tested small molecules do not affect apoSOD1 thermal stability but do influence its tertiary interactions and dynamics, as evidenced by combined biophysical analysis and proteolytic susceptibility. Moreover, these compounds influence apoSOD1 aggregation, decreasing nucleation time and promoting the formation of larger and less soluble aggregates, and in some cases polymeric assemblies apparently composed by spherical species resembling the soluble native protein. We conclude that some components of the ALS metabolome that shape the chemical environment in the CSF may influence apoSOD1 conformers and aggregation.
Figures



Similar articles
-
Calcium ions promote superoxide dismutase 1 (SOD1) aggregation into non-fibrillar amyloid: a link to toxic effects of calcium overload in amyotrophic lateral sclerosis (ALS)?J Biol Chem. 2013 Aug 30;288(35):25219-25228. doi: 10.1074/jbc.M113.470740. Epub 2013 Jul 16. J Biol Chem. 2013. PMID: 23861388 Free PMC article.
-
Disease-related changes in the cerebrospinal fluid metabolome in amyotrophic lateral sclerosis detected by GC/TOFMS.PLoS One. 2011 Apr 4;6(4):e17947. doi: 10.1371/journal.pone.0017947. PLoS One. 2011. PMID: 21483737 Free PMC article.
-
ALS patients with mutations in the SOD1 gene have an unique metabolomic profile in the cerebrospinal fluid compared with ALS patients without mutations.Mol Genet Metab. 2012 Mar;105(3):472-8. doi: 10.1016/j.ymgme.2011.11.201. Epub 2011 Dec 10. Mol Genet Metab. 2012. PMID: 22264771
-
SOD1 aggregation and ALS: role of metallation states and disulfide status.Curr Top Med Chem. 2012;12(22):2560-72. doi: 10.2174/1568026611212220010. Curr Top Med Chem. 2012. PMID: 23339308 Review.
-
How do ALS-associated mutations in superoxide dismutase 1 promote aggregation of the protein?Trends Biochem Sci. 2007 Feb;32(2):78-85. doi: 10.1016/j.tibs.2006.12.005. Epub 2007 Jan 5. Trends Biochem Sci. 2007. PMID: 17208444 Review.
Cited by
-
Probing the kinetic stabilities of Friedreich's ataxia clinical variants using a solid phase GroEL chaperonin capture platform.Biomolecules. 2014 Oct 20;4(4):956-79. doi: 10.3390/biom4040956. Biomolecules. 2014. PMID: 25333765 Free PMC article.
-
"Proteinjury": a universal pathological mechanism mediated by cerebrospinal fluid in neurodegeneration and trauma.Front Cell Dev Biol. 2025 May 20;13:1593122. doi: 10.3389/fcell.2025.1593122. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40463840 Free PMC article. Review.
References
-
- Kato S., Takikawa M., Nakashima K., Hirano A., Cleveland D.W., Kusaka H., Shibata N., Kato M., Nakano I., Ohama E. New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (sod1) gene mutations: Inclusions containing sod1 in neurons and astrocytes. Amyotrophic Lateral Scler. Other Mot. Neuron Disord. 2000;1:163–184. - PubMed
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Shibata N., Asayama K., Hirano A., Kobayashi M. Immunohistochemical study on superoxide dismutases in spinal cords from autopsied patients with amyotrophic lateral sclerosis. Dev. Neurosci. 1996;18:492–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

