Small molecules present in the cerebrospinal fluid metabolome influence superoxide dismutase 1 aggregation
- PMID: 24048249
- PMCID: PMC3794824
- DOI: 10.3390/ijms140919128
Small molecules present in the cerebrospinal fluid metabolome influence superoxide dismutase 1 aggregation
Abstract
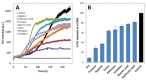
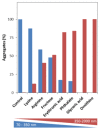
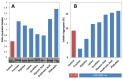
Superoxide dismutase 1 (SOD1) aggregation is one of the pathological markers of amyotrophic lateral sclerosis (ALS), a fatal neurodegenerative disorder. The underlying molecular grounds of SOD1 pathologic aggregation remains obscure as mutations alone are not exclusively the cause for the formation of protein inclusions. Thus, other components in the cell environment likely play a key role in triggering SOD1 toxic aggregation in ALS. Recently, it was found that ALS patients present a specific altered metabolomic profile in the cerebrospinal fluid (CSF) where SOD1 is also present and potentially interacts with metabolites. Here we have investigated how some of these small molecules affect apoSOD1 structure and aggregation propensity. Our results show that as co-solvents, the tested small molecules do not affect apoSOD1 thermal stability but do influence its tertiary interactions and dynamics, as evidenced by combined biophysical analysis and proteolytic susceptibility. Moreover, these compounds influence apoSOD1 aggregation, decreasing nucleation time and promoting the formation of larger and less soluble aggregates, and in some cases polymeric assemblies apparently composed by spherical species resembling the soluble native protein. We conclude that some components of the ALS metabolome that shape the chemical environment in the CSF may influence apoSOD1 conformers and aggregation.
Figures



References
-
- Kato S., Takikawa M., Nakashima K., Hirano A., Cleveland D.W., Kusaka H., Shibata N., Kato M., Nakano I., Ohama E. New consensus research on neuropathological aspects of familial amyotrophic lateral sclerosis with superoxide dismutase 1 (sod1) gene mutations: Inclusions containing sod1 in neurons and astrocytes. Amyotrophic Lateral Scler. Other Mot. Neuron Disord. 2000;1:163–184. - PubMed
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Shibata N., Asayama K., Hirano A., Kobayashi M. Immunohistochemical study on superoxide dismutases in spinal cords from autopsied patients with amyotrophic lateral sclerosis. Dev. Neurosci. 1996;18:492–498. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

