Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma
- PMID: 24048333
- PMCID: PMC3834029
- DOI: 10.1158/1078-0432.CCR-13-0596
Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma
Abstract
Purpose: New prognostic markers to guide treatment decisions in early stage non-small cell lung cancer are necessary to improve patient outcomes. In this report, we assess the utility of a predefined mRNA expression signature of cell-cycle progression genes (CCP score) to define 5-year risk of lung cancer-related death in patients with early stage lung adenocarcinoma.
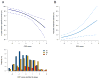
Experimental design: A CCP score was calculated from the mRNA expression levels of 31 proliferation genes in stage I and stage II tumor samples from two public microarray datasets [Director's Consortium (DC) and GSE31210]. The same gene set was tested by quantitative PCR in 381 formalin-fixed paraffin-embedded (FFPE) primary tumors. Association of the CCP score with outcome was assessed by Cox proportional hazards analysis.
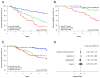
Results: In univariate analysis, the CCP score was a strong predictor of cancer-specific survival in both the Director's Consortium cohort (P = 0.00014; HR = 2.08; 95% CI, 1.43-3.02) and GSE31210 (P = 0.0010; HR = 2.25; 95% CI, 1.42-3.56). In multivariate analysis, the CCP score remained the dominant prognostic marker in the presence of clinical variables (P = 0.0022; HR = 2.02; 95% CI, 1.29-3.17 in Director's Consortium, P = 0.0026; HR = 2.16; 95% CI, 1.32-3.53 in GSE31210). On a quantitative PCR platform, the CCP score maintained highly significant prognostic value in FFPE-derived mRNA from clinical samples in both univariate (P = 0.00033; HR = 2.10; 95% CI, 1.39-3.17) and multivariate analyses (P = 0.0071; HR = 1.92; 95% CI, 1.18-3.10).
Conclusions: The CCP score is a significant predictor of lung cancer death in early stage lung adenocarcinoma treated with surgery and may be a valuable tool in selecting patients for adjuvant treatment.
©2013 AACR.
Conflict of interest statement
Figures
References
-
- Pisters KM, Evans WK, Azzoli CG, Kris MG, Smith CA, Desch CE, et al. Cancer Care Ontario and American Society of Clinical Oncology adjuvant chemotherapy and adjuvant radiation therapy for stages I–IIIA resectable non-small cell lung cancer guideline. J Clin Oncol. 2007;25:5506–18. - PubMed
-
- Crino L, Weder W, van Meerbeeck J, Felip E ESMO Guidelines Working Group. Early stage and locally advanced (non-metastatic) non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2010;21 (Suppl 5):v103–15. - PubMed
-
- Pignon JP, Tribodet H, Scagliotti GV, Douillard JY, Shepherd FA, Stephens RJ, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol. 2008;26:3552–9. - PubMed
-
- Strauss GM, Herndon JE, 2nd, Maddaus MA, Johnstone DW, Johnson EA, Harpole DH, et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–51. - PMC - PubMed
-
- Tsuboi M, Ohira T, Saji H, Miyajima K, Kajiwara N, Uchida O, et al. The present status of postoperative adjuvant chemotherapy for completely resected non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2007;13:73–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

