MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia
- PMID: 24048523
- PMCID: PMC4071620
- DOI: 10.1126/scitranslmed.3006061
MHC class I-associated phosphopeptides are the targets of memory-like immunity in leukemia
Abstract
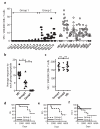
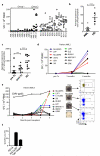
Deregulation of signaling pathways is a hallmark of malignant transformation. Signaling-associated phosphoproteins can be degraded to generate cancer-specific phosphopeptides that are presented by major histocompatibility complex (MHC) class I and II molecules and recognized by T cells; however, the contribution of these phosphoprotein-specific T cells to immune surveillance is unclear. We identified 95 phosphopeptides presented on the surface of primary hematological tumors and normal tissues, including 61 that were tumor-specific. Phosphopeptides were more prevalent on more aggressive and malignant samples. CD8(+) T cell lines specific for these phosphopeptides recognized and killed both leukemia cell lines and human leukocyte antigen-matched primary leukemia cells ex vivo. Notably, healthy individuals showed robust CD8(+) T cell responses against many of these phosphopeptides within the circulating memory compartment. This immunity was significantly reduced or absent in some leukemia patients. This reduction correlated with clinical outcome; however, immunity was restored after allogeneic stem cell transplantation. These results suggest that phosphopeptides may be targets of cancer immune surveillance in humans, and point to their importance for development of vaccine-based and T cell adoptive transfer immunotherapies.
Figures


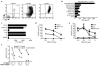

References
-
- Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. - PubMed
-
- Preudhomme C, Guilhot J, Nicolini FE, Guerci-Bresler A, Rigal-Huguet F, Maloisel F, Coiteux V, Gardembas M, Berthou C, Vekhoff A, Rea D, Jourdan E, Allard C, Delmer A, Rousselot P, Legros L, Berger M, Corm S, Etienne G, Roche-Lestienne C, Eclache V, Mahon FX, Guilhot F. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N.Engl.J.Med. 2010;363:2511–2521. - PubMed
-
- Talpaz M, Kantarjian HM, McCredie K, Trujillo JM, Keating MJ, Gutterman JU. Hematologic remission and cytogenetic improvement induced by recombinant human interferon alpha A in chronic myelogenous leukemia. N.Engl.J.Med. 1986;314:1065–1069. - PubMed
-
- Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, Rimm AA, Ringden O, Rozman C, Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. - PubMed
-
- Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, Heim M, Wilmanns W. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–2465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

