Extension of the Caenorhabditis elegans Pharyngeal M1 neuron axon is regulated by multiple mechanisms
- PMID: 24048649
- PMCID: PMC3815062
- DOI: 10.1534/g3.113.008466
Extension of the Caenorhabditis elegans Pharyngeal M1 neuron axon is regulated by multiple mechanisms
Abstract
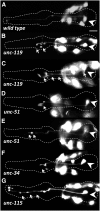
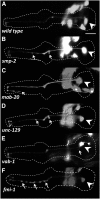
The guidance of axons to their correct targets is a critical step in development. The C. elegans pharynx presents an attractive system to study neuronal pathfinding in the context of a developing organ. The worm pharynx contains relatively few cells and cell types, but each cell has a known lineage and stereotyped developmental patterns. We found that extension of the M1 pharyngeal axon, which spans the entire length of the pharynx, occurs in two distinct phases. The first proximal phase does not require genes that function in axon extension (unc-34, unc-51, unc-115, and unc-119), whereas the second distal phase does use these genes and is guided in part by the adjacent g1P gland cell projection. unc-34, unc-51, and unc-115 had incompletely penetrant defects and appeared to act in conjunction with the g1P cell for distal outgrowth. Only unc-119 showed fully penetrant defects for the distal phase. Mutations affecting classical neuronal guidance cues (Netrin, Semaphorin, Slit/Robo, Ephrin) or adhesion molecules (cadherin, IgCAM) had, at best, weak effects on the M1 axon. None of the mutations we tested affected the proximal phase of M1 elongation. In a forward genetic screen, we isolated nine mutations in five genes, three of which are novel, showing defects in M1, including axon overextension, truncation, or ectopic branching. One of these mutations appeared to affect the generation or differentiation of the M1 neuron. We conclude that M1 axon extension is a robust process that is not completely dependent on any single guidance mechanism.
Keywords: Caenorhabditis elegans; axon; genetics; neuron; pharynx.
Figures




References
-
- Albertson D. G., Thomson J. N., 1976. The pharynx of Caenorhabditis elegans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 275: 299–325 - PubMed
-
- Altun-Gultekin Z., Andachi Y., Tsalik E. L., Pilgrim D., Kohara Y., et al. , 2001. A regulatory cascade of three homeobox genes, ceh-10, ttx-3 and ceh-23, controls cell fate specification of a defined interneuron class in C. elegans. Development 128: 1951–1969 - PubMed
-
- Araujo S. J., Tear G., 2003. Axon guidance mechanisms and molecules: lessons from invertebrates. Nat. Rev. Neurosci. 4: 910–922 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
