Enhanced excitability of thalamic sensory neurons and slow-wave EEG pattern after stimuli that induce spinal long-term potentiation
- PMID: 24048841
- PMCID: PMC6618413
- DOI: 10.1523/JNEUROSCI.2110-13.2013
Enhanced excitability of thalamic sensory neurons and slow-wave EEG pattern after stimuli that induce spinal long-term potentiation
Abstract
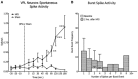
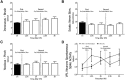
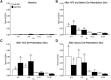
Spinal nociceptive neurons are well known to undergo a process of long-term potentiation (LTP) following conditioning by high-frequency sciatic nerve stimulation (HFS) at intensities recruiting C-fibers. However, little if any information exists as to whether such HFS conditioning that produces spinal LTP affects sensory transmission at supraspinal levels. The present study explored this possibility. Conventional extracellular recording methods were used to examine the consequences of HFS versus sham HFS conditioning on individual wide-dynamic range thalamic neurons located in the ventro-postero-lateral (VPL) nucleus in isoflurane-anesthetized rats. Following HFS, the ongoing firing rate and stimulus-evoked (brush, pinch, sciatic nerve) responses were markedly enhanced as were responses to juxtacellular, microiontophoretic applications of glutamate. These HFS-induced enhancements lasted throughout the recording period. Sham stimuli had no effect on VPL neuron excitability. Cortical electroencephalographic (EEG) wave activities were also measured around HFS in conjunction with VPL neuron recordings. The cortical EEG pattern under baseline conditions consisted of recurring short duration bursts of high-amplitude slow waves followed by longer periods of flat EEG. Following HFS, the EEG shifted to a continuous large-amplitude, slow-wave pattern within the 0.5-8.0 Hz bandwidth lasting throughout the recording period. Sham HFS did not alter EEG activity. Sciatic nerve conditioning at A-δ fiber strength, known to reverse spinal LTP, did not alter enhanced neuronal excitability or the EEG slow-wave pattern induced by HFS. These data support the concept that HFS conditioning of the sciatic nerve, which leads to spinal LTP, is associated with distinct, long-lasting changes in the excitability of neurons comprising thalamocortical networks.
Figures





References
-
- Condés-Lara M, Sanchez-Moreno RM, Omaña-Zapata I. Cortical facilitatory action on centralis lateralis thalamic activity during the development of carrageenin-produced inflammation. Arch Med Res. 1996;27:265–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
