The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity
- PMID: 24048902
- PMCID: PMC3848201
- DOI: 10.4049/jimmunol.1301170
The mitochondrial antiviral protein MAVS associates with NLRP3 and regulates its inflammasome activity
Abstract
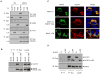
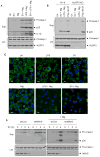
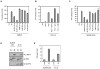
NLRP3 assembles an inflammasome complex that activates caspase-1 upon sensing various danger signals derived from pathogenic infection, tissue damage, and environmental toxins. How NLRP3 senses these various stimuli is still poorly understood, but mitochondria and mitochondrial reactive oxygen species have been proposed to play a critical role in NLRP3 activation. In this article, we provide evidence that the mitochondrial antiviral signaling protein MAVS associates with NLRP3 and facilitates its oligomerization leading to caspase-1 activation. In reconstituted 293T cells, full-length MAVS promoted NLRP3-dependent caspase-1 activation, whereas a C-terminal transmembrane domain-truncated mutant of MAVS (MAVS-ΔTM) did not. MAVS, but not MAVS-ΔTM, interacted with NLRP3 and triggered the oligomerization of NLRP3, suggesting that mitochondrial localization of MAVS and intact MAVS signaling are essential for activating the NLRP3 inflammasome. Supporting this, activation of MAVS signaling by Sendai virus infection promoted NLRP3-dependent caspase-1 activation, whereas knocking down MAVS expression clearly attenuated the activation of NLRP3 inflammasome by Sendai virus in THP-1 and mouse macrophages. Taken together, our results suggest that MAVS facilitates the recruitment of NLRP3 to the mitochondria and may enhance its oligomerization and activation by bringing it in close proximity to mitochondrial reactive oxygen species.
Figures



References
-
- Tschopp J, Schroder K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. - PubMed
-
- Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature. 2012;481:278–286. - PubMed
-
- Aksentijevich I, DP C, Remmers EF, Mueller JL, Le J, Kolodner RD, Moak Z, Chuang M, Austin F, Goldbach-Mansky R, Hoffman HM, Kastner DL. The clinical continuum of cryopyrinopathies: novel CIAS1 mutations in North American patients and a new cryopyrin model. Arthritis Rheum. 2007;56:1273–1285. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

