Pre-activation of the genome integrity checkpoint increases DNA damage tolerance
- PMID: 24049076
- PMCID: PMC3905891
- DOI: 10.1093/nar/gkt820
Pre-activation of the genome integrity checkpoint increases DNA damage tolerance
Abstract
The genome integrity checkpoint is a conserved signaling pathway that is regulated in yeast by the Mec1 (homologous to human ATR) and Rad53 (homologous to human Chk1) kinases. The pathway coordinates a multifaceted response that allows cells to cope with DNA damage and DNA replication stress. The full activation of the checkpoint blocks origin firing, stabilizes replication forks, activates DNA repair proteins and may lead to senescence or apoptosisin higher eukaryotes. We have recently demonstrated that endogenous replication stress can activate the genome integrity checkpoint in budding yeast at a low level that does not go so far as to interfere with cell cycle progression, but it does activate DNA damage-inducible proteins. Here we demonstrate that the low level pre-activation of the checkpoint, either by endogenous replication stress or by the nucleotide-depleting drug hydroxyurea, can increase damage tolerance to multiple DNA-damaging agents. These results may provide new strategies for using the checkpoint to protect normal cells from genotoxic stress.
Figures
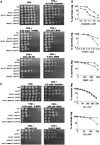
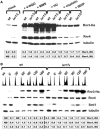

Similar articles
-
Limiting amounts of budding yeast Rad53 S-phase checkpoint activity results in increased resistance to DNA alkylation damage.Nucleic Acids Res. 2006;34(20):5852-62. doi: 10.1093/nar/gkl741. Epub 2006 Oct 24. Nucleic Acids Res. 2006. PMID: 17062626 Free PMC article.
-
Separate roles for the DNA damage checkpoint protein kinases in stabilizing DNA replication forks.Genes Dev. 2008 Jul 1;22(13):1816-27. doi: 10.1101/gad.477208. Genes Dev. 2008. PMID: 18593882 Free PMC article.
-
Ixr1 is required for the expression of the ribonucleotide reductase Rnr1 and maintenance of dNTP pools.PLoS Genet. 2011 May;7(5):e1002061. doi: 10.1371/journal.pgen.1002061. Epub 2011 May 5. PLoS Genet. 2011. PMID: 21573136 Free PMC article.
-
Signaling pathways of replication stress in yeast.FEMS Yeast Res. 2017 Mar 1;17(2). doi: 10.1093/femsyr/fow101. FEMS Yeast Res. 2017. PMID: 27915243 Review.
-
Functional interplay between the oxidative stress response and DNA damage checkpoint signaling for genome maintenance in aerobic organisms.J Microbiol. 2020 Feb;58(2):81-91. doi: 10.1007/s12275-020-9520-x. Epub 2019 Dec 23. J Microbiol. 2020. PMID: 31875928 Review.
Cited by
-
The HMGB Protein KlIxr1, a DNA Binding Regulator of Kluyveromyces lactis Gene Expression Involved in Oxidative Metabolism, Growth, and dNTP Synthesis.Biomolecules. 2021 Sep 21;11(9):1392. doi: 10.3390/biom11091392. Biomolecules. 2021. PMID: 34572607 Free PMC article.
-
Deoxyribonucleotide metabolism, mutagenesis and cancer.Nat Rev Cancer. 2015 Sep;15(9):528-39. doi: 10.1038/nrc3981. Nat Rev Cancer. 2015. PMID: 26299592 Review.
-
The Identification of Genetic Determinants of Methanol Tolerance in Yeast Suggests Differences in Methanol and Ethanol Toxicity Mechanisms and Candidates for Improved Methanol Tolerance Engineering.J Fungi (Basel). 2021 Jan 27;7(2):90. doi: 10.3390/jof7020090. J Fungi (Basel). 2021. PMID: 33513997 Free PMC article.
-
Deoxyribonucleotides as genetic and metabolic regulators.FASEB J. 2014 Sep;28(9):3832-40. doi: 10.1096/fj.14-251249. Epub 2014 Jun 13. FASEB J. 2014. PMID: 24928192 Free PMC article. Review.
-
Alternative Chk1-independent S/M checkpoint in somatic cells that prevents premature mitotic entry.Med Oncol. 2017 Apr;34(4):70. doi: 10.1007/s12032-017-0932-3. Epub 2017 Mar 27. Med Oncol. 2017. PMID: 28349497
References
-
- Hu J, Sun L, Shen F, Chen Y, Hua Y, Liu Y, Zhang M, Hu Y, Wang Q, Xu W, et al. The intra-S phase checkpoint targets Dna2 to prevent stalled replication forks from reversing. Cell. 2012;149:1221–1232. - PubMed
-
- Tercero JA, Diffley JF. Regulation of DNA replication fork progression through damaged DNA by the Mec1/Rad53 checkpoint. Nature. 2001;412:553–557. - PubMed
-
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat. Rev. Mol. Cell Biol. 2010;11:208–219. - PubMed
-
- Lopes M, Cotta-Ramusino C, Pellicioli A, Liberi G, Plevani P, Muzi-Falconi M, Newlon CS, Foiani M. The DNA replication checkpoint response stabilizes stalled replication forks. Nature. 2001;412:557–561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

