TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway
- PMID: 24049142
- PMCID: PMC3840254
- DOI: 10.1152/ajprenal.00182.2013
TGF-β1 stimulates mitochondrial oxidative phosphorylation and generation of reactive oxygen species in cultured mouse podocytes, mediated in part by the mTOR pathway
Abstract
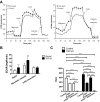
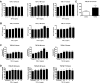
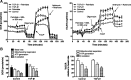
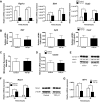
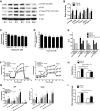
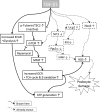
Transforming growth factor (TGF)-β has been associated with podocyte injury; we have examined its effect on podocyte bioenergetics. We studied transformed mouse podocytes, exposed to TGF-β1, using a label-free assay system, Seahorse XF24, which measures oxygen consumption rates (OCR) and extracellular acidification rates (ECAR). Both basal OCR and ATP generation-coupled OCR were significantly higher in podocytes exposed to 0.3-10 ng/ml of TGF-β1 for 24, 48, and 72 h. TGF-β1 (3 ng/ml) increased oxidative capacity 75%, and 96% relative to control after 48 and 72 h, respectively. ATP content was increased 19% and 30% relative to control after a 48- and 72-h exposure, respectively. Under conditions of maximal mitochondrial function, TGF-β1 increased palmitate-driven OCR by 49%. Thus, TGF-β1 increases mitochondrial oxygen consumption and ATP generation in the presence of diverse energy substrates. TGF-β1 did not increase cell number or mitochondrial DNA copy number but did increase mitochondrial membrane potential (MMP), which could explain the OCR increase. Reactive oxygen species (ROS) increased by 32% after TGF-β1 exposure for 48 h. TGF-β activated the mammalian target of rapamycin (mTOR) pathway, and rapamycin reduced the TGF-β1-stimulated increases in OCR, ECAR, ATP generation, cellular metabolic activity, and protein generation. Our data suggest that TGF-β1, acting, in part, via mTOR, increases mitochondrial MMP and OCR, resulting in increased ROS generation and that this may contribute to podocyte injury.
Keywords: TGF-β1; bioenergetics; extracellular acidification rate; mitochondria; oxygen consumption rate; podocyte.
Figures


References
-
- Bishop T, Brand MD. Processes contributing to metabolic depression in hepatopancreas cells from the snail Helix aspersa. J Exp Biol 203: 3603–3612, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

