Acquisition of RGC phenotype in human Müller glia with stem cell characteristics is accompanied by upregulation of functional nicotinic acetylcholine receptors
- PMID: 24049438
- PMCID: PMC3774575
Acquisition of RGC phenotype in human Müller glia with stem cell characteristics is accompanied by upregulation of functional nicotinic acetylcholine receptors
Abstract
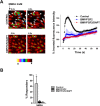
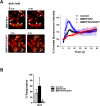
Purpose: Human Müller glia with stem cell characteristics (hMGSCs) can be induced to express genes and proteins of retinal ganglion cells (RGCs) upon in vitro inhibition of Notch-1 activity. However, it is not known whether expression of these markers is accompanied by acquisition of RGC function. This study investigated whether hMGSCs that express RGC markers also display neural functionality, as measured by their intracellular calcium concentration ([Ca(2+)]i) responsiveness following neurotransmitter stimulation in vitro.
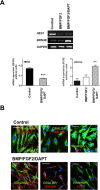
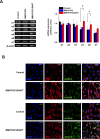
Methods: Changes in mRNA expression of RGC markers and neurotransmitter receptors were assessed either by conventional or quantitative reverse transcription PCR (RT-PCR), while changes in protein levels were confirmed by immunocytochemistry. The [Ca(2+)]i levels were estimated by fluorescence microscopy.
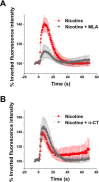
Results: We showed that while undifferentiated hMGSCs displayed a profound elevation of [Ca(2+)]i after stimulation with N-methyl-D-aspartate (NMDA), this was lost following Notch-1 inhibition. Conversely, untreated hMGSCs did not respond to muscarinic receptor stimulation, whereas [Ca(2+)]i was increased in differentiated hMGSCs that expressed RGC precursor markers. Differentiated hMGSC-derived RGCs, but not undifferentiated hMGSCs, responded to stimulation by nicotine with a substantial rise in [Ca(2+)]i, which was inhibited by the α4β2 and α6β2 nicotinic receptor antagonist methyllycaconitine. Notch-1 attenuation not only caused a decrease in the gene expression of the Notch effector HES1 and increased expression of RGC markers, but also an increase in the gene and protein expression of α4 and α6 nicotinic receptor subunits.
Conclusions: These observations suggest that in response to Notch-1 inhibition, hMGSCs differentiate into a population of RGCs that exhibit some of the functionality observed in differentiated RGCs.
Figures
References
-
- Tropepe V, Coles BL, Chiasson BJ, Horsford DJ, Elia AJ, McInnes RR, van der Kooy D. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–6. - PubMed
-
- MacNeil A, Pearson RA, MacLaren RE, Smith AJ, Sowden JC, Ali RR. Comparative analysis of progenitor cells isolated from the iris, pars plana, and ciliary body of the adult porcine eye. Stem Cells. 2007;25:2430–8. - PubMed
-
- Lawrence JM, Singhal S, Bhatia B, Keegan DJ, Reh TA, Luthert PJ, Khaw PT, Limb GA. MIO-M1 cells and similar Müller glial cell lines derived from adult human retina exhibit neural stem cell characteristics. Stem Cells. 2007;25:2033–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
