(11)C-meta-hydroxyephedrine PET/CT imaging allows in vivo study of adaptive thermogenesis and white-to-brown fat conversion
- PMID: 24049730
- PMCID: PMC3773828
- DOI: 10.1016/j.molmet.2013.04.002
(11)C-meta-hydroxyephedrine PET/CT imaging allows in vivo study of adaptive thermogenesis and white-to-brown fat conversion
Abstract
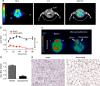
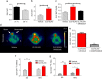
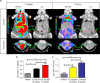
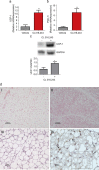
Several lines of evidence suggest that novel pharmacological approaches aimed at converting white adipose tissue (WAT) into brown adipose tissue (BAT) may represent an effective therapeutic strategy for obesity and related disorders. ((18))F-fluorodeoxyglucose ((18)F-FDG) is the only positron emission tomography (PET) tracer commonly used to study BAT function, and so far no functional tools have been described to investigate in vivo white-to-brown fat conversion. In this report, we show that the PET tracer (11)C-meta-hydroxyephedrine ((11)C-MHED, a norepinephrine analogue) is a useful tool to investigate the sympathetic nervous system (SNS) activity in BAT of lean and dietary obese mice. Moreover, we demonstrate that (11)C-MHED is a specific marker of the SNS-mediated thermogenesis in typical BAT depots, and that this tracer can detect in vivo WAT to BAT conversion.
Keywords: Brown adipose tissue; PET/CT imaging; Sympathetic activity.
Figures
References
-
- Rothwell N.J., Stock M.J. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. - PubMed
-
- Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. - PubMed
-
- Feldmann H.M., Golozoubova V., Cannon B., Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9:203–209. - PubMed
-
- Cederberg A., Grønning L.M., Ahrén B., Taskén K., Carlsson P., Enerbäck S. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. - PubMed
-
- Bartelt A., Bruns O.T., Reimer R., Hohenberg H., Ittrich H., Peldschus K. Brown adipose tissue activity controls triglyceride clearance. Nature Medicine. 2011;17:200–205. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

