Bilirubin neurotoxicity in preterm infants: risk and prevention
- PMID: 24049745
- PMCID: PMC3775137
- DOI: 10.4103/2249-4847.116402
Bilirubin neurotoxicity in preterm infants: risk and prevention
Abstract
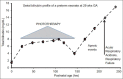
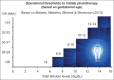
Hemolytic conditions in preterm neonates, including Rhesus (Rh) disease, can lead to mortality and long-term impairments due to bilirubin neurotoxicity. Universal access to Rh immunoprophylaxis, coordinated perinatal-neonatal care, and effective phototherapy has virtually eliminated the risk of kernicterus in many countries. In the absence of jaundice due to isoimmunization and without access to phototherapy or exchange transfusion (in 1955), kernicterus was reported at 10.1%, 5.5%, and 1.2% in babies <30, 31-32, and 33-34 wks gestational age, respectively. Phototherapy initiated at 24±12 hr effectively prevented hyperbilirubinemia in infants <2,000 g even in the presence of hemolysis. This approach (in 1985) reduced exchange transfusions from 23.9% to 4.8%. Now with 3 decades of experience in implementing effective phototherapy, the need for exchange transfusions has virtually been eliminated. However, bilirubin neurotoxicity continues to be associated with prematurity alone. The ability to better predict this risk, other than birthweight and gestation, has been elusive. Objective tests such as total bilirubin, unbound or free bilirubin, albumin levels, and albumin-bilirubin binding, together with observations of concurrent hemolysis, sepsis, and rapid rate of bilirubin rise have been considered, but their individual or combined predictive utility has yet to be refined. The disruptive effects of immaturity, concurrent neonatal disease, cholestasis, use of total parenteral nutrition or drugs that alter bilirubin-binding abilities augment the clinical risk of neurotoxicity. Current management options rely on the "fine-tuning" of each infant's exposure to beneficial antioxidants and avoidance of silent neurotoxic properties of bilirubin navigated within the safe spectrum of operational thresholds demarcated by experts.
Keywords: Bilirubin neurotoxicity; hyperbilirubinemia; jaundice; kernicterus; preterm.
Conflict of interest statement
Figures
References
-
- American Academy of Pediatrics. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114:297–316. - PubMed
-
- Maisels MJ, Bhutani VK, Bogen D, Newman TB, Stark AR, Watchko JF. Hyperbilirubinemia in the newborn infant > or = 35 weeks’ gestation: An update with clarifications. Pediatrics. 2009;124:1193–8. - PubMed
-
- Ip S, Lau J, Chung M, Kulig J, Sege R, Glicken S, et al. Hyperbilirubinemia and kernicterus: 50 years later. Pediatrics. 2004;114:263–4. - PubMed
-
- Maisels MJ, Watchko JF, Bhutani VK, Stevenson DK. An approach to the management of hyperbilirubinemia in the preterm infant less than 35 weeks of gestation. J Perinatol. 2012;32:660–4. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources