Oral Nystatin Versus Intravenous Fluconazole as Neonatal Antifungal Prophylaxis: Non-inferiority Trial
- PMID: 24049751
- PMCID: PMC3775143
- DOI: 10.4103/2249-4847.116408
Oral Nystatin Versus Intravenous Fluconazole as Neonatal Antifungal Prophylaxis: Non-inferiority Trial
Abstract
Background: Fluconazole has shown to be effective in reducing both colonization and invasive Candida infection (ICI) in ELBW neonates; we conducted a randomized trial to compare oral nystatin with intravenous fluconazole for prophylaxis against invasive Candidiasis in high risk neonates.
Materials and methods: By using SPSS, preterm less than 30 weeks gestation and/or birth weight 1200 grams or less assigned to receive either intravenous Fluconazole (6 mg/kg q72 hr for 1(st) week then q48 h for 6 wks) or oral Nystatin (100,000 unit q8 hr for 6 wks). The medications commenced at one week of age after obtaining the base line investigations and check for Candida colonization by urine culture and rectal swab; subsequently all lab work and the clinical data were monitered regularly. Risk factors were assessed. The data collected prospectively looking for primary end point the invasive Candida infection (ICI) and 2 ndry outcomes include medication safety, tolerance and cost.
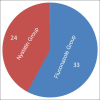
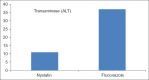
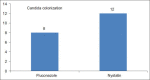
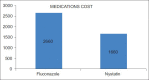
Results: 65 neonate randomly assigned however only 57 neonates comleted the study 33/57 (57%) to intravenous fluconazole group and 24/57 (42%) to oral nystatin group. No differences in birth weights Nystatin (1.15 Kg) Fluconazole (1.01 Kg), gender males (26/57), female (32/57), Gestational age (29.28 vs l28.22) or risk factors between the two groups. Rectal swab Colonization occurred in 2/24 (8%) in Nystatin group and 4/33 (12%) in the Fluconazole group, but none of the neonates developed ICI or side effects, although in the Fluconazole group transient transaminase elevation 2SD standard deviation above the mean was observed. Central line duration was 2 SD above the mean for fluconazole group, The cost of the Fluconazole treated group (7,581 SAR) 106.4 US/pt double the cost of Nystatin treated group (3,375 SAR) 50 US/pt.
Conclusion: Intravenous Fluconazole and oral Nystatin at the prophylactic doses are equally effective and safe in preventing (ICI) in preterm neonates, however oral Nystatin is readily available, easily administered with lower cost per neonate.
Keywords: Candida; fluconazole; neonatal; nystatin; prophylaxis.
Conflict of interest statement
Figures
References
-
- Makhoul IR, Sujov P, Smolkin T, Lusky A, Reichman B. Epidemiological, clinical, and microbiological characteristics of late-onset sepsis among very low birth weight infants in Israel: A national survey. Pediatrics. 2002;109:34–9. - PubMed
-
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. - PubMed
-
- Kossoff EH, Buescher ES, Karlowicz MG. Candidemia in a neonatal intensive care unit: Trends during fifteen years and clinical features of 111 cases. Pediatr Infect Dis J. 1998;17:504–8. - PubMed
-
- Saiman L, Ludington E, Pfaller M, Rangel-Frausto S, Wiblin RT, Dawson J, et al. Risk factors for candidemia in Neonatal Intensive Care Unit patients. The National Epidemiology of Mycosis Survey study group. Pediatr Infect Dis J. 2000;19:319–24. - PubMed
-
- Karlowicz MG, Rowen JL, Barnes-Eley ML, Burke BL, Lawson ML, Bendel CM, et al. The role of birth weight and gestational age in distinguishing extremely low birth weight infants at high risk of developing candidemia from infants at low risk: A multicenter study. Pediatr Res. 2002;51:301A.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous