Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes
- PMID: 24050525
- PMCID: PMC3820380
- DOI: 10.1017/S0033583513000061
Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: insights from Torpedo postsynaptic membranes
Abstract
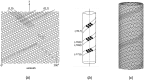
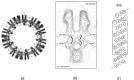
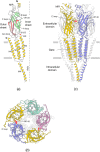
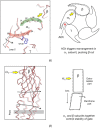
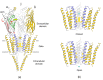
The nicotinic acetylcholine (ACh) receptor, at the neuromuscular junction, is a neurotransmitter-gated ion channel that has been fine-tuned through evolution to transduce a chemical signal into an electrical signal with maximum efficiency and speed. It is composed from three similar and two identical polypeptide chains, arranged in a ring around a narrow membrane pore. Central to the design of this assembly is a hydrophobic gate in the pore, more than 50 Å away from sites in the extracellular domain where ACh binds. Although the molecular properties of the receptor have been explored intensively over the last few decades, only recently have structures emerged revealing its complex architecture and illuminating how ACh entering the binding sites opens the distant gate. Postsynaptic membranes isolated from the (muscle-derived) electric organ of the Torpedo ray have underpinned most of the structural studies: the membranes form tubular vesicles having receptors arranged on a regular surface lattice, which can be imaged directly in frozen physiological solutions. Advances in electron crystallographic techniques have also been important, enabling analysis of the closed- and open-channel forms of the receptor in unreacted tubes or tubes reacted briefly with ACh. The structural differences between these two forms show that all five subunits participate in a concerted conformational change communicating the effect of ACh binding to the gate, but that three of them (αγ, β and δ) play a dominant role. Flexing of oppositely facing pore-lining α-helices is the principal motion determining the closed/open state of the gate. These results together with the findings of biochemical, biophysical and other structural studies allow an integrated description of the receptor and of its mode of action at the synapse.
Figures





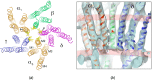

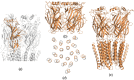



References
-
- Adrian A. M., Dubochet J., Lepault J. & Mcdowall A. W. (1984). Cryo-electron microscopy of viruses. Nature 308, 32–36 - PubMed
-
- Andreeva I. E., Nirthanan S., Cohen J. B. & Pedersen S. E. (2006). Site specificity of agonist-induced opening and desensitisation of the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 45, 195–204 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

