Ligand-induced stabilization of the aptamer terminal helix in the add adenine riboswitch
- PMID: 24051105
- PMCID: PMC3851719
- DOI: 10.1261/rna.040493.113
Ligand-induced stabilization of the aptamer terminal helix in the add adenine riboswitch
Abstract
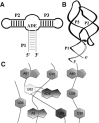
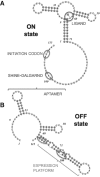
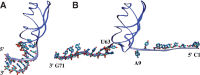
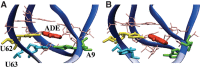
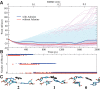
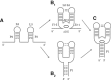
Riboswitches are structured mRNA elements that modulate gene expression. They undergo conformational changes triggered by highly specific interactions with sensed metabolites. Among the structural rearrangements engaged by riboswitches, the forming and melting of the aptamer terminal helix, the so-called P1 stem, is essential for genetic control. The structural mechanisms by which this conformational change is modulated upon ligand binding mostly remain to be elucidated. Here, we used pulling molecular dynamics simulations to study the thermodynamics of the P1 stem in the add adenine riboswitch. The P1 ligand-dependent stabilization was quantified in terms of free energy and compared with thermodynamic data. This comparison suggests a model for the aptamer folding in which direct P1-ligand interactions play a minor role on the conformational switch when compared with those related to the ligand-induced aptamer preorganization.
Keywords: P1 stem; RNA aptamer; adenine riboswitch; free energy calculation; molecular dynamics simulation.
Figures


Similar articles
-
Molecular dynamics simulation of the binding process of ligands to the add adenine riboswitch aptamer.Phys Rev E. 2019 Aug;100(2-1):022412. doi: 10.1103/PhysRevE.100.022412. Phys Rev E. 2019. PMID: 31574664
-
Role of the adenine ligand on the stabilization of the secondary and tertiary interactions in the adenine riboswitch.J Mol Biol. 2010 Mar 12;396(5):1422-38. doi: 10.1016/j.jmb.2009.12.024. Epub 2009 Dec 21. J Mol Biol. 2010. PMID: 20026131 Free PMC article.
-
Using reweighted pulling simulations to characterize conformational changes in riboswitches.Methods Enzymol. 2015;553:139-62. doi: 10.1016/bs.mie.2014.10.055. Epub 2015 Feb 3. Methods Enzymol. 2015. PMID: 25726464
-
Riboswitch Mechanisms for Regulation of P1 Helix Stability.Int J Mol Sci. 2024 Oct 4;25(19):10682. doi: 10.3390/ijms251910682. Int J Mol Sci. 2024. PMID: 39409011 Free PMC article. Review.
-
Linking aptamer-ligand binding and expression platform folding in riboswitches: prospects for mechanistic modeling and design.Wiley Interdiscip Rev RNA. 2015 Nov-Dec;6(6):631-50. doi: 10.1002/wrna.1300. Epub 2015 Sep 11. Wiley Interdiscip Rev RNA. 2015. PMID: 26361734 Free PMC article. Review.
Cited by
-
Binding free energy decomposition and multiple unbinding paths of buried ligands in a PreQ1 riboswitch.PLoS Comput Biol. 2021 Nov 12;17(11):e1009603. doi: 10.1371/journal.pcbi.1009603. eCollection 2021 Nov. PLoS Comput Biol. 2021. PMID: 34767553 Free PMC article.
-
Ligand-mediated and tertiary interactions cooperatively stabilize the P1 region in the guanine-sensing riboswitch.PLoS One. 2017 Jun 22;12(6):e0179271. doi: 10.1371/journal.pone.0179271. eCollection 2017. PLoS One. 2017. PMID: 28640851 Free PMC article.
-
Structures of riboswitch RNA reaction states by mix-and-inject XFEL serial crystallography.Nature. 2017 Jan 12;541(7636):242-246. doi: 10.1038/nature20599. Epub 2016 Nov 14. Nature. 2017. PMID: 27841871 Free PMC article.
-
RNA Structural Dynamics As Captured by Molecular Simulations: A Comprehensive Overview.Chem Rev. 2018 Apr 25;118(8):4177-4338. doi: 10.1021/acs.chemrev.7b00427. Epub 2018 Jan 3. Chem Rev. 2018. PMID: 29297679 Free PMC article. Review.
-
Elastic network models for RNA: a comparative assessment with molecular dynamics and SHAPE experiments.Nucleic Acids Res. 2015 Sep 3;43(15):7260-9. doi: 10.1093/nar/gkv708. Epub 2015 Jul 17. Nucleic Acids Res. 2015. PMID: 26187990 Free PMC article.
References
-
- Batey RT, Gilbert SD, Montange RK 2004. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432: 411–415 - PubMed
-
- Bayly CI, Cieplak P, Cornell W, Kollman PA 1993. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: The RESP model. J Phys Chem 97: 10269–10280
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
