The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy
- PMID: 24051278
- PMCID: PMC3908775
- DOI: 10.1016/j.nbd.2013.09.003
The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy
Abstract
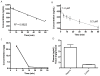
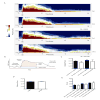
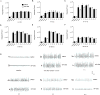
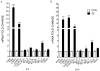
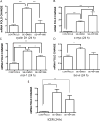
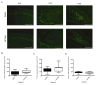
Pilocarpine-induced status epilepticus (SE), which results in temporal lobe epilepsy (TLE) in rodents, activates the JAK/STAT pathway. In the current study, we evaluate whether brief exposure to a selective inhibitor of the JAK/STAT pathway (WP1066) early after the onset of SE affects the severity of SE or reduces later spontaneous seizure frequency via inhibition of STAT3-regulated gene transcription. Rats that received systemic WP1066 or vehicle at the onset of SE were continuously video-EEG monitored during SE and for one month to assess seizure frequency over time. Protein and/or mRNA levels for pSTAT3, and STAT3-regulated genes including: ICER, Gabra1, c-myc, mcl-1, cyclin D1, and bcl-xl were evaluated in WP1066 and vehicle-treated rats during stages of epileptogenesis to determine the acute effects of WP1066 administration on SE and chronic epilepsy. WP1066 (two 50mg/kg doses) administered within the first hour after onset of SE results in transient inhibition of pSTAT3 and long-term reduction in spontaneous seizure frequency. WP1066 alters the severity of chronic epilepsy without affecting SE or cell death. Early WP1066 administration reduces known downstream targets of STAT3 transcription 24h after SE including cyclin D1 and mcl-1 levels, known for their roles in cell-cycle progression and cell survival, respectively. These findings uncover a potential effect of the JAK/STAT pathway after brain injury that is physiologically important and may provide a new therapeutic target that can be harnessed for the prevention of epilepsy development and/or progression.
Keywords: B-cell lymphoma 2; B-cell lymphoma-extra large; Cyclin D1; G1/S-specific cyclin-D1 (encoded by CCND1); ICER; JAK; Janus kinase; STAT; Signal Transducer and Activator of Transcription; VEGF; bcl-2; bcl-xl; c-myc; cellular regulatory gene that codes for a transcription factor; induced myeloid leukemia cell differentiation protein; inducible cAMP Early Repressor protein; mcl-1; vascular endothelial growth factor.
© 2013.
Figures
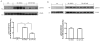
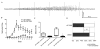
References
-
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–5. - PubMed
-
- Azuma Y, et al. Particular nuclear transcription factors responsive to systemic administration of kainic acid in murine brain. Neurochem Int. 1996;29:289–99. - PubMed
-
- Balish M, et al. Seizure frequency in intractable partial epilepsy: a statistical analysis. Epilepsia. 1991;32:642–9. - PubMed
-
- Bauer J, Burr W. Course of chronic focal epilepsy resistant to anticonvulsant treatment. Seizure. 2001;10:239–46. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

