REEPing the benefits of an animal model of hereditary spastic paraplegia
- PMID: 24051371
- PMCID: PMC3784552
- DOI: 10.1172/JCI72324
REEPing the benefits of an animal model of hereditary spastic paraplegia
Abstract
The hereditary spastic paraplegias (HSPs) are characterized by spasticity of the leg muscles due to axonal degeneration of corticospinal neurons. Beetz et al. report that the core motor phenotype and axonal pathology of HSPs are recapitulated in mice lacking the HSP-associated gene Reep1. REEP1 is shown to regulate ER structure in motor cortex neurons. The Reep1 knockout mouse should be a very useful model in which to study the mechanisms of progressive axon loss in HSPs and other disorders.
Figures
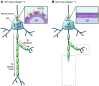
Comment on
-
A spastic paraplegia mouse model reveals REEP1-dependent ER shaping.J Clin Invest. 2013 Oct;123(10):4273-82. doi: 10.1172/JCI65665. Epub 2013 Sep 24. J Clin Invest. 2013. PMID: 24051375 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

