A spastic paraplegia mouse model reveals REEP1-dependent ER shaping
- PMID: 24051375
- PMCID: PMC3784524
- DOI: 10.1172/JCI65665
A spastic paraplegia mouse model reveals REEP1-dependent ER shaping
Erratum in
- J Clin Invest. 2014 Jun 2;124(6):2809
Abstract
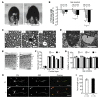
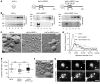
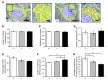
Axonopathies are a group of clinically diverse disorders characterized by the progressive degeneration of the axons of specific neurons. In hereditary spastic paraplegia (HSP), the axons of cortical motor neurons degenerate and cause a spastic movement disorder. HSP is linked to mutations in several loci known collectively as the spastic paraplegia genes (SPGs). We identified a heterozygous receptor accessory protein 1 (REEP1) exon 2 deletion in a patient suffering from the autosomal dominantly inherited HSP variant SPG31. We generated the corresponding mouse model to study the underlying cellular pathology. Mice with heterozygous deletion of exon 2 in Reep1 displayed a gait disorder closely resembling SPG31 in humans. Homozygous exon 2 deletion resulted in the complete loss of REEP1 and a more severe phenotype with earlier onset. At the molecular level, we demonstrated that REEP1 is a neuron-specific, membrane-binding, and membrane curvature-inducing protein that resides in the ER. We further show that Reep1 expression was prominent in cortical motor neurons. In REEP1-deficient mice, these neurons showed reduced complexity of the peripheral ER upon ultrastructural analysis. Our study connects proper neuronal ER architecture to long-term axon survival.
Figures



Comment in
-
REEPing the benefits of an animal model of hereditary spastic paraplegia.J Clin Invest. 2013 Oct;123(10):4134-6. doi: 10.1172/JCI72324. Epub 2013 Sep 24. J Clin Invest. 2013. PMID: 24051371 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

