Dendritic epidermal T cells regulate skin antimicrobial barrier function
- PMID: 24051381
- PMCID: PMC3784546
- DOI: 10.1172/JCI70064
Dendritic epidermal T cells regulate skin antimicrobial barrier function
Abstract
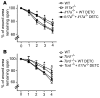
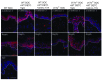
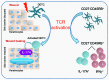
The epidermis, the outer layer of the skin, forms a physical and antimicrobial shield to protect the body from environmental threats. Skin injury severely compromises the epidermal barrier and requires immediate repair. Dendritic epidermal T cells (DETC) reside in the murine epidermis where they sense skin injury and serve as regulators and orchestrators of immune responses. Here, we determined that TCR stimulation and skin injury induces IL-17A production by a subset of DETC. This subset of IL-17A-producing DETC was distinct from IFN-γ producers, despite similar surface marker profiles. Functionally, blocking IL-17A or genetic deletion of IL-17A resulted in delayed wound closure in animals. Skin organ cultures from Tcrd-/-, which lack DETC, and Il17a-/- mice both exhibited wound-healing defects. Wound healing was fully restored by the addition of WT DETC, but only partially restored by IL-17A-deficient DETC, demonstrating the importance of IL-17A to wound healing. Following skin injury, DETC-derived IL-17A induced expression of multiple host-defense molecules in epidermal keratinocytes to promote healing. Together, these data provide a mechanistic link between IL-17A production by DETC, host-defense, and wound-healing responses in the skin. These findings establish a critical and unique role of IL-17A-producing DETC in epidermal barrier function and wound healing.
Figures





References
-
- Braff MH, Gallo RL. Antimicrobial peptides: an essential component of the skin defensive barrier. Curr Top Microbiol Immunol. 2006;306:91–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

