Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids
- PMID: 24051516
- PMCID: PMC4141680
- DOI: 10.1177/2211068213504296
Transfer, imaging, and analysis plate for facile handling of 384 hanging drop 3D tissue spheroids
Abstract
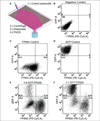
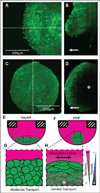
Three-dimensional culture systems bridge the experimental gap between in vivo and in vitro physiology. However, nonstandardized formation and limited downstream adaptability of 3D cultures have hindered mainstream adoption of these systems for biological applications, especially for low- and moderate-throughput assays commonly used in biomedical research. Here we build on our recent development of a 384-well hanging drop plate for spheroid culture to design a complementary spheroid transfer and imaging (TRIM) plate. The low-aspect ratio wells of the TRIM plate facilitated high-fidelity, user-independent, contact-based collection of hanging drop spheroids. Using the TRIM plate, we demonstrated several downstream analyses, including bulk tissue collection for flow cytometry, high-resolution low working-distance immersion imaging, and timely reagent delivery for enzymatic studies. Low working-distance multiphoton imaging revealed a cell type-dependent, macroscopic spheroid structure. Unlike ovarian cancer spheroids, which formed loose, disk-shaped spheroids, human mammary fibroblasts formed tight, spherical, and nutrient-limited spheroids. Beyond the applications we describe here, we expect the hanging drop spheroid plate and complementary TRIM plate to facilitate analyses of spheroids across the spectrum of throughput, particularly for bulk collection of spheroids and high-content imaging.
Keywords: 3D tissue spheroids; and hanging drop spheroids; high-throughput imaging.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.T. has licensed the 384 hanging drop array technology to 3D Biomatrix and also has stock options.
Figures

References
-
- Zhou Y, Arai T, Horiguchi Y, et al. Multi-Parameter Analyses of Three-Dimensionally Cultured Tumor Spheroids Based on Respiratory Activity and Comprehensive Gene-Expression Profiles. Anal. Biochem. 2013;439:187–193. - PubMed
-
- Kelm JM, Fussenegger M. Microscale Tissue Engineering Using Gravity-Enforced Cell Assembly. Trends Biotechnol. 2004;22:195–202. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

