Role of the C-terminal domain in the structure and function of tetrameric sodium channels
- PMID: 24051986
- PMCID: PMC3791462
- DOI: 10.1038/ncomms3465
Role of the C-terminal domain in the structure and function of tetrameric sodium channels
Abstract
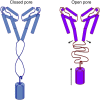
Voltage-gated sodium channels have essential roles in electrical signalling. Prokaryotic sodium channels are tetramers consisting of transmembrane (TM) voltage-sensing and pore domains, and a cytoplasmic carboxy-terminal domain. Previous crystal structures of bacterial sodium channels revealed the nature of their TM domains but not their C-terminal domains (CTDs). Here, using electron paramagnetic resonance (EPR) spectroscopy combined with molecular dynamics, we show that the CTD of the NavMs channel from Magnetococcus marinus includes a flexible region linking the TM domains to a four-helix coiled-coil bundle. A 2.9 Å resolution crystal structure of the NavMs pore indicates the position of the CTD, which is consistent with the EPR-derived structure. Functional analyses demonstrate that the coiled-coil domain couples inactivation with channel opening, and is enabled by negatively charged residues in the linker region. A mechanism for gating is proposed based on the structure, whereby splaying of the bottom of the pore is possible without requiring unravelling of the coiled-coil.
Figures





References
-
- Hille B. Ionic Channels of Excitable Membranes Sinauer Associates Inc.: Sunderland, MA, (2001).
-
- Noda M. et al. Expression of functional sodium channels from cloned cDNA. Nature 322, 826–828 (1986). - PubMed
-
- Nurani G. et al. Tetrameric bacterial sodium channels: characterization of structure, stability, and drug binding. Biochemistry 47, 8114–8121 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

