Antitumor immune responses induced by ionizing irradiation and further immune stimulation
- PMID: 24052136
- PMCID: PMC11028436
- DOI: 10.1007/s00262-013-1474-y
Antitumor immune responses induced by ionizing irradiation and further immune stimulation
Abstract
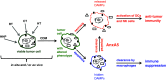
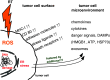
The therapy of cancer emerged as multimodal treatment strategy. The major mode of action of locally applied radiotherapy (RT) is the induction of DNA damage that triggers a network of events that finally leads to tumor cell cycle arrest and cell death. Along with this, RT modifies the phenotype of the tumor cells and their microenvironment. Either may contribute to the induction of specific and systemic antitumor immune responses. The latter are boosted when additional immune therapy (IT) is applied at distinct time points during RT. We will focus on therapy-induced necrotic tumor cell death that is immunogenic due to the release of damage-associated molecular patterns. Immune-mediated distant bystander (abscopal) effects of RT when combined with dendritic cell-based IT and the role of fractionation of radiation in the induction of immunogenic tumor cell death will be discussed. Autologous whole-tumor-cell-based vaccines generated by high hydrostatic pressure technology will be introduced and the influence of cytokines and the immune modulator AnnexinA5 on the ex vivo generated or in situ therapy-induced vaccine efficacy will be outlined. RT should be regarded as immune adjuvant for metastatic disease and as a tool for the generation of an in situ vaccine when applied at distinct fractionation doses or especially in combination with IT to generate immune memory against the tumor. To identify the most beneficial combination and chronology of RT with IT is presumably one of the biggest challenges of innovative tumor research and therapies.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

