GPCR: G protein complexes--the fundamental signaling assembly
- PMID: 24052187
- PMCID: PMC3845202
- DOI: 10.1007/s00726-013-1593-y
GPCR: G protein complexes--the fundamental signaling assembly
Abstract
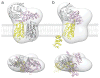
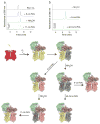
G protein coupled receptors (GPCR) constitute the largest group of cell surface receptors that transmit various signals across biological membranes through the binding and activation of heterotrimeric G proteins, which amplify the signal and activate downstream effectors leading to the biological responses. Thus, the first critical step in this signaling cascade is the interaction between receptor and its cognate G protein. Understanding this critical event at the molecular level is of high importance because abnormal function of GPCRs is associated with many diseases. Thus, these receptors are targets for drug development.
Conflict of interest statement
The author declares that she has no conflict of interst.
Figures
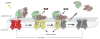

References
-
- Albizu L, Cottet M, Kralikova M, Stoev S, Seyer R, Brabet I, Roux T, Bazin H, Bourrier E, Lamarque L, Breton C, Rives ML, Newman A, Javitch J, Trinquet E, Manning M, Pin JP, Mouillac B, Durroux T. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat Chem Biol. 2010;6 (8):587–594. doi: 10.1038/nchembio.396. - DOI - PMC - PubMed
-
- Baneres JL, Parello J. Structure-based analysis of GPCR function: evidence for a novel pentameric assembly between the dimeric leukotriene B4 receptor BLT1 and the G-protein. Journal of molecular biology. 2003;329 (4):815–829. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

