Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword?
- PMID: 24052632
- PMCID: PMC3785270
- DOI: 10.1681/ASN.2012080857
Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword?
Abstract
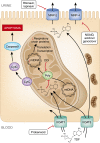
Tenofovir disoproxil fumarate (TDF), the first nucleotidic inhibitor of HIV reverse transcription, became available in 2001. It has been extensively used worldwide and is now the most prescribed antiretroviral (ARV) drug. Its high antiviral activity and favorable metabolic profile are responsible for its success. Furthermore, TDF has been associated with other ARVs to form new combined antiretroviral treatments in only one tablet once-a-day, which increases treatment adherence. Fears of potential nephrotoxicity that tenofovir would have in common with two other drugs from the same family (adefovir, used to treat hepatitis B, and cidofovir, used to treat cytomegalovirus infections) were alleviated by the early clinical trials. Yet, in 2001, the first case of TDF-induced acute nephrotoxicity was published. Numerous cases have been published since then, and it is now established that TDF presents a tubular toxicity risk. Some facilitating factors have been identified, such as co-prescription of didanosine or boosted protease inhibitor, preexisting CKD, low body weight, and associated diabetes mellitus. Conversely, whether TDF is nephrotoxic in the long term is a highly debated question. Some studies suggest a decreased GFR when TDF is prescribed for a long period, while others indicate that TDF is safe for the kidneys even after many years of use. Here we review the differences in patient characteristics, study designs, and measured outcomes that can possibly explain these conflicting findings. We conclude with rational recommendation for appropriate TDF prescription.
Figures

References
-
- Gilead Science 2006 annual report on marketed products. 2007. Available at: http://www.gilead.ca/AR2006/hiv_aids.php Accessed September 9, 2013
-
- Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F: Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 359: 2442–2455, 2008 - PubMed
-
- U.S. Food and Drug Administration. Viread. 2008. Modified indication. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021356s025lbl.pdf Accessed September 9, 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

