Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases
- PMID: 24052633
- PMCID: PMC3839551
- DOI: 10.1681/ASN.2013020173
Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases
Abstract
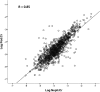
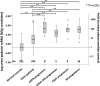
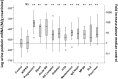
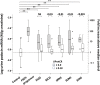
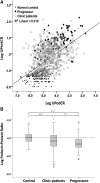
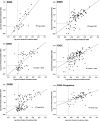
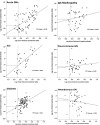
Model systems demonstrate that progression to ESRD is driven by progressive podocyte depletion (the podocyte depletion hypothesis) and can be noninvasively monitored through measurement of urine pellet podocyte mRNAs. To test these concepts in humans, we analyzed urine pellet mRNAs from 358 adult and pediatric kidney clinic patients and 291 controls (n=1143 samples). Compared with controls, urine podocyte mRNAs increased 79-fold (P<0.001) in patients with biopsy-proven glomerular disease and a 50% decrease in kidney function or progression to ESRD. An independent cohort of patients with Alport syndrome had a 23-fold increase in urinary podocyte mRNAs (P<0.001 compared with controls). Urinary podocyte mRNAs increased during active disease but returned to baseline on disease remission. Furthermore, urine podocyte mRNAs increased in all categories of glomerular disease evaluated, but levels ranged from high to normal, consistent with individual patient variability in the risk for progression. In contrast, urine podocyte mRNAs did not increase in polycystic kidney disease. The association between proteinuria and podocyturia varied markedly by glomerular disease type: a high correlation in minimal-change disease and a low correlation in membranous nephropathy. These data support the podocyte depletion hypothesis as the mechanism driving progression in all human glomerular diseases, suggest that urine pellet podocyte mRNAs could be useful for monitoring risk for progression and response to treatment, and provide novel insights into glomerular disease pathophysiology.
Figures

References
-
- Wiggins RC: The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int 71: 1205–1214, 2007 - PubMed
-
- Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 - PubMed
-
- Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R: Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001 - PubMed
-
- Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 - PubMed
-
- Kriz W, LeHir M: Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R01 DK046073/DK/NIDDK NIH HHS/United States
- UL1TR000433/TR/NCATS NIH HHS/United States
- 5T32DK7378-34/DK/NIDDK NIH HHS/United States
- U54 DK083912/DK/NIDDK NIH HHS/United States
- UL1RR024986/RR/NCRR NIH HHS/United States
- P30 DK081943/DK/NIDDK NIH HHS/United States
- T32 DK065517/DK/NIDDK NIH HHS/United States
- T32 DK007378/DK/NIDDK NIH HHS/United States
- T32DK065517/DK/NIDDK NIH HHS/United States
- DK R0146073/DK/NIDDK NIH HHS/United States
- P30DK081943/DK/NIDDK NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

