The role of the NFAT signaling pathway in retinal neovascularization
- PMID: 24052639
- PMCID: PMC3809948
- DOI: 10.1167/iovs.13-12183
The role of the NFAT signaling pathway in retinal neovascularization
Abstract
Purpose: The purpose of the present study was to investigate the role of nuclear factor of activated T cells (NFAT), a transcription factor downstream of VEGF, in angiogenic cell behaviors of human retinal microvascular endothelial cells (HRMEC), and to assess the efficacy of NFAT signaling inhibitors in a rat model of oxygen-induced retinopathy (OIR).
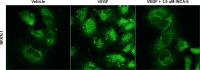
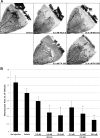
Methods: Human retinal microvascular endothelial cells were treated with VEGF in the presence or absence of the NFAT inhibitor of NFAT-calcineurin association-6 (INCA-6), and NFAT translocation was evaluated using immunocytochemistry (ICC). Human retinal microvascular endothelial cells were treated with increasing doses of INCA-6, and cell proliferation and tube formation were assessed. Rats subjected to OIR were administered increasing doses of INCA-6 or the CN inhibitor FK-506, and the retinal neovascular area was measured.
Results: Nuclear factor of activated T-cells c1 was translocated to the nucleus of HRMEC treated with VEGF, and INCA-6 treatment blocked translocation. Inhibitor of NFAT-calcineurin association-6inhibited HRMEC proliferation and tube formation in a dose-dependent manner. Both INCA-6 and FK-506 treatment significantly reduced pathologic neovascularization in OIR.
Conclusions: This investigation has demonstrated that in HRMEC, NFATc1 is activated downstream of VEGF signaling and NFAT signaling plays a key role in angiogenic cell behaviors. In addition, NFAT inhibition is shown to be highly efficacious in an OIR model. These findings indicate that the NFAT signaling pathway may serve as a suitable therapeutic target for the treatment of neovascular eye disease.
Keywords: NFAT; neovascularization; oxygen-induced retinopathy; retinal microvascular endothelial cells; transcription factors.
Figures


References
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992; 267: 10931–10934 - PubMed
-
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell. 1996; 87: 1153–1155 - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003; 9: 653–660 - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995; 1: 27–31 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

