Facile Access to Ring-Fused Aminals via Direct α-Amination of Secondary Amines with ortho-Aminobenzaldehydes. Synthesis of Vasicine, Deoxyvasicine, Deoxyvasicinone, Mackinazolinone and Ruteacarpine
- PMID: 24052668
- PMCID: PMC3774014
Facile Access to Ring-Fused Aminals via Direct α-Amination of Secondary Amines with ortho-Aminobenzaldehydes. Synthesis of Vasicine, Deoxyvasicine, Deoxyvasicinone, Mackinazolinone and Ruteacarpine
Abstract
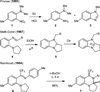
Secondary amines undergo redox-neutral reactions with aminobenzaldehydes under conventional and microwave heating to furnish polycyclic aminals via amine α-amination/N-alkylation. This unique α-functionalization reaction proceeds without the involvement of transition metals or other additives. The resulting aminal products are precursors for various quinazolinone alkaloids and their analogues.
Keywords: Aminals; C–H bond functionalization; Friedländer condensation; Quinazolinone alkaloids; Redox Isomerization.
Figures















References
-
-
Selected reviews on amine α-functionalization: Murahashi S-I. Angew. Chem., Int. Ed. Engl. 1995;34:2443. Doye S. Angew. Chem. Int. Ed. 2001;40:3351. Tobisu M, Chatani N. Angew. Chem. Int. Ed. 2006;45:1683. Campos KR. Chem. Soc. Rev. 2007;36:1069. Murahashi S-I, Zhang D. Chem. Soc. Rev. 2009;37:1490. Li C-J. Acc. Chem. Res. 2009;42:335. Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem. Eur. J. 2010;16:2654. Yoo W-J, Li C-J. Top. Curr. Chem. 2010;292:281. Yeung CS, Dong VM. Chem. Rev. 2011;111:1215. Sun C-L, Li B-J, Shi Z-J. Chem. Rev. 2011;111:1293. Liu C, Zhang H, Shi W, Lei AW. Chem. Rev. 2011;111:1780. Wendlandt AE, Suess AM, Stahl SS. Angew. Chem. Int. Ed. 2011;50:11062. Klussmann M, Jones KM. Synlett. 2012;23:159. Zhang C, Tang CH, Jiao N. Chem. Soc. Rev. 2012;41:3464. Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem. Eur. J. 2012;18:10092. Pan SC. Beilstein J. Org. Chem. 2012;8:1374.
-
-
-
Pinnow J. Ber. 1895;28:3039. See also: Ruiz MDR, Vasella A. Helv. Chim. Acta. 2011;94:785.
-
-
- Meth-Cohn O, Suschitzky H. Adv. Heterocycl. Chem. 1972;14:211.
- Verboom W, Reinhoudt DN. Recl. Trav. Chim. Pays-Bas. 1990;109:311.
- Meth-Cohn O. Adv. Heterocycl. Chem. 1996;65:1.
- Quintela JM. Recent Res. Devel. Org. Chem. 2003;7:259.
- Matyus P, Elias O, Tapolcsanyi P, Polonka-Balint A, Halasz-Dajka B. Synthesis. 2006:2625.
-
-
Meth-Cohn O, Naqui MA. Chem. Commun. 1967:1157. See also: Ryabukhin SV, Plaskon AS, Volochnyuk DM, Shivanyuk AN, Tolmachev AA. J. Org. Chem. 2007;72:7417. Che X, Zheng L, Dang Q, Bai X. Synlett. 2008:2373.
-
Grants and funding
LinkOut - more resources
Full Text Sources
