Necrosis avidity: a newly discovered feature of hypericin and its preclinical applications in necrosis imaging
- PMID: 24052807
- PMCID: PMC3776218
- DOI: 10.7150/thno.6650
Necrosis avidity: a newly discovered feature of hypericin and its preclinical applications in necrosis imaging
Abstract
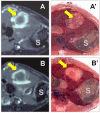
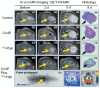
Hypericin has been widely studied as a potent photosensitizer for photodynamic therapy in both preclinical and clinical settings. Recently, hypericin has also been discovered to have a specific avidity for necrotic tissue. This affinity is also observed in a series of radiolabeled derivatives of hypericin, including [(123)I]iodohypericin, [(124)I]iodohypericin, and [(131)I]iodohypericin. Hypericin, along with other necrosis-avid contrast agents, has been investigated for use in noninvasively targeting necrotic tissues in numerous disorders. Potential clinical applications of hypericin include the identification of acute myocardial infarction, evaluation of tissue viability, assessment of therapeutic responses to treatments, and interventional procedures for solid tumors. The mechanisms of necrosis avidity in hypericin remain to be fully elucidated, although several hypotheses have been suggested. In particular, it has been proposed that the necrosis avidity of hypericin is compound specific; for instance, cholesterol, phosphatidylserine, or phosphatidylethanolamine components in the phospholipid bilayer of cellular membranes may be the major targets for its observed selectivity. Further investigations are needed to identify the specific binding moiety that is responsible for the necrosis avidity of hypericin.
Keywords: avidity; hypericin; necrosis.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Kacerovska D, Pizinger K, Majer F. et al. Photodynamic therapy of nonmelanoma skin cancer with topical hypericum perforatum extract--a pilot study. Photochem Photobiol. 2008;84:779–785. - PubMed
-
- Miskovsky P. Hypericin--a new antiviral and antitumor photosensitizer: mechanism of action and interaction with biological macromolecules. Curr Drug Targets. 2002;3:55–84. - PubMed
-
- Saw CL, Olivo M, Soo KC. et al. Spectroscopic characterization and photobleaching kinetics of hypericin-N-methyl pyrrolidone formulations. Photochem Photobiol Sci. 2006;5:1018–1023. - PubMed
-
- Olivo M, Lau W, Manivasager V. et al. Novel photodynamic diagnosis of bladder cancer: ex vivo fluorescence cytology using hypericin. Int J Oncol. 2003;23:1501–1504. - PubMed
-
- Olivo M, Du HY, Bay BH. Hypericin lights up the way for the potential treatment of nasopharyngeal cancer by photodynamic therapy. Curr Clin Pharmacol. 2006;1:217–222. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

