New triple therapy for chronic hepatitis C: real life clinical experience in a community setting
- PMID: 24052911
- PMCID: PMC3764547
New triple therapy for chronic hepatitis C: real life clinical experience in a community setting
Abstract
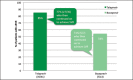
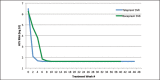
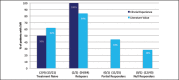
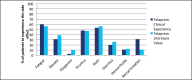
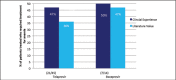
Recent advances in treatment of chronic hepatitis C virus have improved significantly due to the introduction of two new protease inhibitors-telaprevir and boceprevir. In combination with the previous standard of care, peginterferon and ribavirin, telaprevir and boceprevir have demonstrated improved sustained virologic response rates for HCV genotype 1 patients by approximately 30%. The purpose of this study was to assess the validity of large clinical trial data with respect to efficacy and side effects in a community setting in Honolulu, Hawai'i. This retrospective study was performed by reviewing the charts of 59 chronic HCV patients who were started on triple therapy from July 1, 2011 to July 7, 2012. Sustained virologic response was attained by 73% of patients treated with telaprevir and 46% of patients treated with boceprevir respectively. Our clinical experience with telaprevir demonstrates that SVR rates are compatible with published literature values. Rates of SVR in our cohort were also similar to those reported in cirrhotic patients - about 50%. Due to small number of patients treated with a boceprevir-based regimen, it is difficult to compare our experience with pivotal trial experience. The side effect profiles for the two protease inhibitors were similar to the literature values except for more rectal irritation and a higher incidence and severity of anemia on telaprevir therapy in the clinical setting. While not intended to be conclusive, our study demonstrates that clinical trial data are largely compatible with the outcomes obtained in our community setting.
Keywords: HCV; Incivek; Victrelis; boceprevir; community experience; real clinical setting; telaprevir; triple therapy.
Figures


References
-
- Centers of Disease Control, author. Hepatitic C Information for Health Professionals. [April 27, 2013]. http://www.cdc.gov/hepatitis/hcv.
-
- Brown RS. Hepatitis C and liver transplantation. Nature. 2005 Aug 18;436(7053):973–978. - PubMed
-
- Ly KN, Xing J, Klevens RM, Jiles RB, Ward JW, Holmberg SD. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012 Feb 21;156(4):271–278. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

