The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1
- PMID: 24052938
- PMCID: PMC3757744
- DOI: 10.1016/j.stemcr.2013.04.001
The interfollicular epidermis of adult mouse tail comprises two distinct cell lineages that are differentially regulated by Wnt, Edaradd, and Lrig1
Abstract
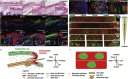
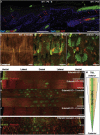
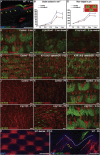
Current models of how mouse tail interfollicular epidermis (ife) is maintained overlook the coexistence of two distinct terminal differentiation programs: parakeratotic (scale) and orthokeratotic (interscale). lineage tracing and clonal analysis revealed that scale and interscale are maintained by unipotent cells in the underlying basal layer, with scale progenitors dividing more rapidly than interscale progenitors. Although scales are pigmented and precisely aligned with hair follicles, melanocytes and follicles were not necessary for scale differentiation. Epidermal Wnt signaling was required for scale enlargement during development and for postnatal maintenance of scale-interscale boundaries. Loss of Edaradd inhibited ventral scale formation, whereas loss of Lrig1 led to scale enlargement and fusion. In wild-type skin, Lrig1 was not expressed in IFE but was selectively upregulated in dermal fibroblasts underlying the interscale. We conclude that the different IFE differentiation compartments are maintained by distinct stem cell populations and are regulated by epidermal and dermal signals.
Figures


References
-
- Arwert E.N., Hoste E., Watt F.M. Epithelial stem cells, wound healing and cancer. Nat. Rev. Cancer. 2012;12:170–180. - PubMed
-
- Braun K.M., Niemann C., Jensen U.B., Sundberg J.P., Silva-Vargas V., Watt F.M. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development. 2003;130:5241–5255. - PubMed
-
- Cable J., Jackson I.J., Steel K.P. Mutations at the W locus affect survival of neural crest-derived melanocytes in the mouse. Mech. Dev. 1995;50:139–150. - PubMed
-
- Carroll J.M., Romero M.R., Watt F.M. Suprabasal integrin expression in the epidermis of transgenic mice results in developmental defects and a phenotype resembling psoriasis. Cell. 1995;83:957–968. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

