Functional vascular endothelium derived from human induced pluripotent stem cells
- PMID: 24052946
- PMCID: PMC3757754
- DOI: 10.1016/j.stemcr.2013.06.007
Functional vascular endothelium derived from human induced pluripotent stem cells
Abstract
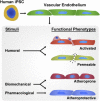
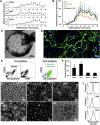
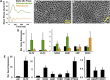
Vascular endothelium is a dynamic cellular interface that displays a unique phenotypic plasticity. This plasticity is critical for vascular function and when dysregulated is pathogenic in several diseases. Human genotype-phenotype studies of endothelium are limited by the unavailability of patient-specific endothelial cells. To establish a cellular platform for studying endothelial biology, we have generated vascular endothelium from human induced pluripotent stem cells (iPSCs) exhibiting the rich functional phenotypic plasticity of mature primary vascular endothelium. These endothelial cells respond to diverse proinflammatory stimuli, adopting an activated phenotype including leukocyte adhesion molecule expression, cytokine production, and support for leukocyte transmigration. They maintain dynamic barrier properties responsive to multiple vascular permeability factors. Importantly, biomechanical or pharmacological stimuli can induce pathophysiologically relevant atheroprotective or atheroprone phenotypes. Our results demonstrate that iPSC-derived endothelium possesses a repertoire of functional phenotypic plasticity and is amenable to cell-based assays probing endothelial contributions to inflammatory and cardiovascular diseases.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

