Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia
- PMID: 24053644
- PMCID: PMC3961841
- DOI: 10.1089/ars.2013.5219
Nrf2 regulates angiogenesis: effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia
Abstract
Aims: Nuclear factor E2-related factor 2 (Nrf2), a key cytoprotective transcription factor, regulates also proangiogenic mediators, interleukin-8 and heme oxygenase-1 (HO-1). However, hitherto its role in blood vessel formation was modestly examined. Particularly, although Nrf2 was shown to affect hematopoietic stem cells, it was not tested in bone marrow-derived proangiogenic cells (PACs). Here we investigated angiogenic properties of Nrf2 in PACs, endothelial cells, and inflammation-related revascularization.
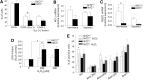
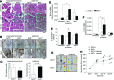
Results: Treatment of endothelial cells with angiogenic cytokines increased nuclear localization of Nrf2 and induced expression of HO-1. Nrf2 activation stimulated a tube network formation, while its inhibition decreased angiogenic response of human endothelial cells, the latter effect reversed by overexpression of HO-1. Moreover, lack of Nrf2 attenuated survival, proliferation, migration, and angiogenic potential of murine PACs and affected angiogenic transcriptome in vitro. Additionally, angiogenic capacity of PAC Nrf2(-/-) in in vivo Matrigel assay and PAC mobilization in response to hind limb ischemia of Nrf2(-/-) mice were impaired. Despite that, restoration of blood flow in Nrf2-deficient ischemic muscles was better and accompanied by increased oxidative stress and inflammatory response. Accordingly, the anti-inflammatory agent etodolac tended to diminish blood flow in the Nrf2(-/-) mice.
Innovation: Identification of a novel role of Nrf2 in angiogenic signaling of endothelial cells and PACs.
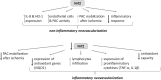
Conclusion: Nrf2 contributes to angiogenic potential of both endothelial cells and PACs; however, its deficiency increases muscle blood flow under tissue ischemia. This might suggest a proangiogenic role of inflammation in the absence of Nrf2 in vivo, concomitantly undermining the role of PACs in such conditions.
Figures







References
-
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, and Lawley TJ. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 99: 683–690, 1992 - PubMed
-
- Afonyushkin T, Oskolkova OV, Philippova M, Resink TJ, Erne P, Binder BR, and Bochkov VN. Oxidized phospholipids regulate expression of ATF4 and VEGF in endothelial cells via NRF2-dependent mechanism: novel point of convergence between electrophilic and unfolded protein stress pathways. Arterioscler Thromb Vasc Biol 30: 1007–1013, 2010 - PubMed
-
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, and Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med 9: 1370–1376, 2003 - PubMed
-
- Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, and Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 - PubMed
-
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, and Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85: 221–228, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

