Catecholamine metabolites affected by the copper-dependent enzyme dopamine-beta-hydroxylase provide sensitive biomarkers for early diagnosis of menkes disease and viral-mediated ATP7A gene therapy
- PMID: 24054147
- PMCID: PMC6421562
- DOI: 10.1016/B978-0-12-411512-5.00011-7
Catecholamine metabolites affected by the copper-dependent enzyme dopamine-beta-hydroxylase provide sensitive biomarkers for early diagnosis of menkes disease and viral-mediated ATP7A gene therapy
Abstract
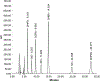
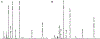
Menkes disease is a lethal X-linked recessive disorder of copper metabolism caused by mutations in ATP7A, a copper-transporting ATPase with diverse and important biological functions. Partial deficiency of dopamine-beta-hydroxylase is a biochemical hallmark of this illness due to the normal role of ATP7A in delivery of copper as an enzymatic cofactor. We exploited this fact to develop a diagnostic test for Menkes disease, which proved highly sensitive and specific. The assay has enabled early identification of affected patients, leading to enhanced survival and improved neurodevelopment after early copper treatment, including some completely normal outcomes. In preclinical efforts to develop improved therapies for patients with non-copper-responsive ATP7A mutations, we used brain-directed adeno-associated viral gene therapy to rescue a murine model of the disease. Statistically significant improvement in brain catechol ratios correlated with enhanced survival, and cerebrospinal fluid catechols represent candidate surrogate markers of treatment effect in a future gene therapy clinical trial.
Keywords: Adeno-associated virus; Catechol ratios; Copper transport; Dopamine-beta-hydroxylase; Gene therapy; Menkes disease; Newborn screening.
© 2013 Elsevier Inc. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
Figures

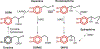
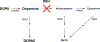

References
-
- Biaggioni I, & Robertson D (1987).Endogenous restoration of noradrenaline by precursor therapy in dopamine-beta-hydroxylase deficiency. Lancet, 2(8569), 1170–1172. - PubMed
-
- Chelly J, Tümer Z, Tønnesen T, Petterson A, Ishikawa-Brush Y, Tommerup N, et al. (1993). Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nature Genetics, 3, 14–19. - PubMed
-
- Christodoulou J, Danks DM, Sarkar B, Baerlocher KE, Casey R, Horn N, et al. (1998). Early treatment of Menkes disease with parenteral copper-histidine: Long-term follow-up of four treated patients. American Journal of Medical Genetics, 76(2), 154–164. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

