Cardiac matrix: a clue for future therapy
- PMID: 24055000
- PMCID: PMC4111554
- DOI: 10.1016/j.bbadis.2013.09.004
Cardiac matrix: a clue for future therapy
Abstract
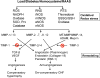
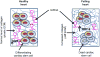
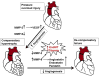
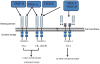
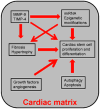
Cardiac muscle is unique because it contracts ceaselessly throughout the life and is highly resistant to fatigue. The marvelous nature of the cardiac muscle is attributed to its matrix that maintains structural and functional integrity and provides ambient micro-environment required for mechanical, cellular and molecular activities in the heart. Cardiac matrix dictates the endothelium myocyte (EM) coupling and contractility of cardiomyocytes. The matrix metalloproteinases (MMPs) and their tissue inhibitor of metalloproteinases (TIMPs) regulate matrix degradation that determines cardiac fibrosis and myocardial performance. We have shown that MMP-9 regulates differential expression of micro RNAs (miRNAs), calcium cycling and contractility of cardiomyocytes. The differential expression of miRNAs is associated with angiogenesis, hypertrophy and fibrosis in the heart. MMP-9, which is involved in the degradation of cardiac matrix and induction of fibrosis, is also implicated in inhibition of survival and differentiation of cardiac stem cells (CSC). Cardiac matrix is distinct because it renders mechanical properties and provides a framework essential for differentiation of cardiac progenitor cells (CPC) into specific lineage. Cardiac matrix regulates myocyte contractility by EM coupling and calcium transients and also directs miRNAs required for precise regulation of continuous and synchronized beating of cardiomyocytes that is indispensible for survival. Alteration in the matrix homeostasis due to induction of MMPs, altered expression of specific miRNAs or impaired signaling for contractility of cardiomyocytes leads to catastrophic effects. This review describes the mechanisms by which cardiac matrix regulates myocardial performance and suggests future directions for the development of treatment strategies in cardiovascular diseases.
Keywords: APT; Angiogenesis; CPC; CSC; CVD; DNA methyl transferase; DNMT; ECM; Heart; MMP; MicroRNA; ROS; SERCA; Stem cell; TIMP; VEGF; acute pulmonary thromboembolism; cardiac progenitor cell; cardiac stem cell; cardiovascular disease; extracellular matrix; matrix metalloproteinase; miRNA; reactive oxygen species; sarco endoplasmic reticulum ca(2+)ATPase; tissue inhibitor of MMP; vascular endothelial growth factor.
© 2013.
Figures
Similar articles
-
Alcohol modulation of cardiac matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs favors collagen accumulation.Alcohol Clin Exp Res. 2014 Feb;38(2):448-56. doi: 10.1111/acer.12239. Epub 2013 Aug 19. Alcohol Clin Exp Res. 2014. PMID: 24033327 Free PMC article.
-
Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling.Arch Physiol Biochem. 2012 Feb;118(1):31-42. doi: 10.3109/13813455.2011.635660. Epub 2011 Dec 19. Arch Physiol Biochem. 2012. PMID: 22181043 Free PMC article. Review.
-
MMP-9 gene ablation and TIMP-4 mitigate PAR-1-mediated cardiomyocyte dysfunction: a plausible role of dicer and miRNA.Cell Biochem Biophys. 2010 Jul;57(2-3):67-76. doi: 10.1007/s12013-010-9084-1. Cell Biochem Biophys. 2010. PMID: 20422465 Free PMC article.
-
Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling.Cardiovasc Res. 2000 May;46(2):214-24. doi: 10.1016/s0008-6363(00)00003-1. Cardiovasc Res. 2000. PMID: 10773225 Review.
-
Interplay of angiotensin II and angiotensin(1-7) in the regulation of matrix metalloproteinases of human cardiocytes.Exp Physiol. 2008 May;93(5):599-612. doi: 10.1113/expphysiol.2007.041830. Epub 2008 Feb 22. Exp Physiol. 2008. PMID: 18296491
Cited by
-
TFAM overexpression reduces pathological cardiac remodeling.Mol Cell Biochem. 2019 Apr;454(1-2):139-152. doi: 10.1007/s11010-018-3459-9. Epub 2018 Oct 23. Mol Cell Biochem. 2019. PMID: 30353496 Free PMC article.
-
Docosahexaenoic acid reverses angiotensin II-induced RECK suppression and cardiac fibroblast migration.Cell Signal. 2014 May;26(5):933-41. doi: 10.1016/j.cellsig.2014.01.005. Epub 2014 Jan 19. Cell Signal. 2014. PMID: 24447911 Free PMC article.
-
Decellularized matrices for cardiovascular tissue engineering.Am J Stem Cells. 2014 Mar 13;3(1):1-20. eCollection 2014. Am J Stem Cells. 2014. PMID: 24660110 Free PMC article. Review.
-
Role of ADMA in the pathogenesis of microvascular complications in type 2 diabetes mellitus.Front Endocrinol (Lausanne). 2023 Apr 21;14:1183586. doi: 10.3389/fendo.2023.1183586. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37152974 Free PMC article. Review.
-
Stable, covalent attachment of laminin to microposts improves the contractility of mouse neonatal cardiomyocytes.ACS Appl Mater Interfaces. 2014 Sep 10;6(17):15516-26. doi: 10.1021/am5042324. Epub 2014 Aug 26. ACS Appl Mater Interfaces. 2014. PMID: 25133578 Free PMC article.
References
-
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
-
- Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy SCIPIO: initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

