Characterization of the α- and β-mannosidases of Porphyromonas gingivalis
- PMID: 24056103
- PMCID: PMC3837954
- DOI: 10.1128/JB.00898-13
Characterization of the α- and β-mannosidases of Porphyromonas gingivalis
Abstract
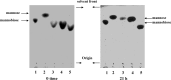
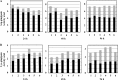
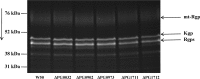
Mannose is an important sugar in the biology of the Gram-negative bacterium Porphyromonas gingivalis. It is a major component of the oligosaccharides attached to the Arg-gingipain cysteine proteases, the repeating units of an acidic lipopolysaccharide (A-LPS), and the core regions of both types of LPS produced by the organism (O-LPS and A-LPS) and a reported extracellular polysaccharide (EPS) isolated from spent culture medium. The organism occurs at inflamed sites in periodontal tissues, where it is exposed to host glycoproteins rich in mannose, which may be substrates for the acquisition of mannose by P. gingivalis. Five potential mannosidases were identified in the P. gingivalis W83 genome that may play a role in mannose acquisition. Four mannosidases were characterized in this study: PG0032 was a β-mannosidase, whereas PG0902 and PG1712 were capable of hydrolyzing p-nitrophenyl α-d-mannopyranoside. PG1711 and PG1712 were α-1 → 3 and α-1 → 2 mannosidases, respectively. No enzyme function could be assigned to PG0973. α-1 → 6 mannobiose was not hydrolyzed by P. gingivalis W50. EPS present in the culture supernatant was shown to be identical to yeast mannan and a component of the medium used for culturing P. gingivalis and was resistant to hydrolysis by mannosidases. Synthesis of O-LPS and A-LPS and glycosylation of the gingipains appeared to be unaffected in all mutants. Thus, α- and β-mannosidases of P. gingivalis are not involved in the harnessing of mannan/mannose from the growth medium for these biosynthetic processes. P. gingivalis grown in chemically defined medium devoid of carbohydrate showed reduced α-mannosidase activity (25%), suggesting these enzymes are environmentally regulated.
Figures


Similar articles
-
Identification of the linkage between A-polysaccharide and the core in the A-lipopolysaccharide of Porphyromonas gingivalis W50.J Bacteriol. 2015 May;197(10):1735-46. doi: 10.1128/JB.02562-14. Epub 2015 Mar 2. J Bacteriol. 2015. PMID: 25733619 Free PMC article.
-
Generation of lys-gingipain protease activity in Porphyromonas gingivalis W50 is independent of Arg-gingipain protease activities.Microbiology (Reading). 2000 Aug;146 ( Pt 8):1933-1940. doi: 10.1099/00221287-146-8-1933. Microbiology (Reading). 2000. PMID: 10931897
-
Expression of Arg-Gingipain RgpB is required for correct glycosylation and stability of monomeric Arg-gingipain RgpA from Porphyromonas gingivalis W50.Infect Immun. 2005 Aug;73(8):4864-78. doi: 10.1128/IAI.73.8.4864-4878.2005. Infect Immun. 2005. PMID: 16041000 Free PMC article.
-
Host cell-surface proteins as substrates of gingipains, the main proteases of Porphyromonas gingivalis.Biol Chem. 2018 Nov 27;399(12):1353-1361. doi: 10.1515/hsz-2018-0215. Biol Chem. 2018. PMID: 29927743 Review.
-
Molecular genetics of Porphyromonas gingivalis: gingipains and other virulence factors.Curr Protein Pept Sci. 2003 Dec;4(6):389-95. doi: 10.2174/1389203033486983. Curr Protein Pept Sci. 2003. PMID: 14683425 Review.
Cited by
-
Type II Secretion-Dependent Aminopeptidase LapA and Acyltransferase PlaC Are Redundant for Nutrient Acquisition during Legionella pneumophila Intracellular Infection of Amoebas.mBio. 2018 Apr 17;9(2):e00528-18. doi: 10.1128/mBio.00528-18. mBio. 2018. PMID: 29666285 Free PMC article.
-
Comparative gene expression analysis of planktonic Porphyromonas gingivalis ATCC 33277 in the presence of a growing biofilm versus planktonic cells.BMC Microbiol. 2019 Mar 12;19(1):58. doi: 10.1186/s12866-019-1423-9. BMC Microbiol. 2019. PMID: 30866810 Free PMC article.
-
Identification of the linkage between A-polysaccharide and the core in the A-lipopolysaccharide of Porphyromonas gingivalis W50.J Bacteriol. 2015 May;197(10):1735-46. doi: 10.1128/JB.02562-14. Epub 2015 Mar 2. J Bacteriol. 2015. PMID: 25733619 Free PMC article.
-
Sequential processing of mannose-containing glycans by two α-mannosidases from Solitalea canadensis.Glycoconj J. 2016 Apr;33(2):159-68. doi: 10.1007/s10719-016-9651-9. Epub 2016 Feb 11. Glycoconj J. 2016. PMID: 26864077
-
Crosstalk of Cellulose and Mannan Perception Pathways Leads to Inhibition of Cellulase Production in Several Filamentous Fungi.mBio. 2019 Jul 2;10(4):e00277-19. doi: 10.1128/mBio.00277-19. mBio. 2019. PMID: 31266859 Free PMC article.
References
-
- Curtis MA, Aduse-Opoku J, Rangarajan M. 2001. Cysteine proteases of Porphyromonas gingivalis. Crit. Rev. Oral Biol. Med. 12:192–216 - PubMed
-
- Paramonov N, Bailey D, Rangarajan M, Hashim A, Kelly G, Curtis MA, Hounsell EF. 2001. Structural analysis of the polysaccharide from the lipopolysaccharide of Porphyromonas gingivalis strain W50. Eur. J. Biochem. 268:4698–4707 - PubMed
-
- Paramonov N, Rangarajan M, Hashim A, Gallagher A, Aduse-Opoku J, Slaney JM, Hounsell E, Curtis MA. 2005. Structural analysis of a novel anionic polysaccharide from Porphyromonas gingivalis strain W50 related to Arg-gingipain glycans. Mol. Microbiol. 58:847–863 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

