Paramyxovirus activation and inhibition of innate immune responses
- PMID: 24056173
- PMCID: PMC3940258
- DOI: 10.1016/j.jmb.2013.09.015
Paramyxovirus activation and inhibition of innate immune responses
Abstract
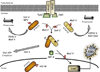
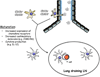
Paramyxoviruses represent a remarkably diverse family of enveloped nonsegmented negative-strand RNA viruses, some of which are the most ubiquitous disease-causing viruses of humans and animals. This review focuses on paramyxovirus activation of innate immune pathways, the mechanisms by which these RNA viruses counteract these pathways, and the innate response to paramyxovirus infection of dendritic cells (DC). Paramyxoviruses are potent activators of extracellular complement pathways, a first line of defense that viruses must face during natural infections. We discuss mechanisms by which these viruses activate and combat complement to delay neutralization. Once cells are infected, virus replication drives type I interferon (IFN) synthesis that has the potential to induce a large number of antiviral genes. Here we describe four approaches by which paramyxoviruses limit IFN induction: by limiting synthesis of IFN-inducing aberrant viral RNAs, through targeted inhibition of RNA sensors, by providing viral decoy substrates for cellular kinase complexes, and through direct blocking of the IFN promoter. In addition, paramyxoviruses have evolved diverse mechanisms to disrupt IFN signaling pathways. We describe three general mechanisms, including targeted proteolysis of signaling factors, sequestering cellular factors, and upregulation of cellular inhibitors. DC are exceptional cells with the capacity to generate adaptive immunity through the coupling of innate immune signals and T cell activation. We discuss the importance of innate responses in DC following paramyxovirus infection and their consequences for the ability to mount and maintain antiviral T cells.
Keywords: DC; ISG; ORF; WT; dendritic cells; innate immunity; interferon; interferon-stimulated gene; moDC; monocyte-derived DC; open reading frame; pDC; paramyxovirus; plasmacytoid DC; wild type.
© 2013.
Figures





References
-
- Lamb RA, Parks GD. Paramyxoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Wolters Kluwer and Lippincott Williams and Wilkins; 2013. pp. 957–995.
-
- Rubin SA, Sauder CJ, Carbone KM. Mumps virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Wolters Kluwer and Lippincott Williams and Wilkins; 2013. pp. 1024–1041.
-
- Griffin DE. Measles virus. In: Knipe DM, Howley PM, editors. Fields Virology. 6th ed. Philadelphia, PA: Wolters Kluwer and Lippincott Williams and Wilkins; 2013. pp. 1042–1069.
-
- Parks GD, Manuse MJ, Johnson JJ. The parainfluenza virus simian virus 5. In: Samal S, editor. The Biology of Paramyxoviruses. Norfolk, UK: Caister Academic Press; 2011. pp. 37–68.
-
- Collins PL. Human respiratory syncytial virus. In: Samal S, editor. The Biology of Paramyxoviruses. Norfolk, UK: Caister Academic Press; 2011. pp. 341–410.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

