Dental pulp tissue engineering in full-length human root canals
- PMID: 24056227
- PMCID: PMC3797540
- DOI: 10.1177/0022034513505772
Dental pulp tissue engineering in full-length human root canals
Abstract
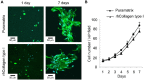
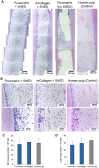
The clinical translation of stem-cell-based dental pulp regeneration will require the use of injectable scaffolds. Here, we tested the hypothesis that stem cells from exfoliated deciduous teeth (SHED) can generate a functional dental pulp when injected into full-length root canals. SHED survived and began to express putative markers of odontoblastic differentiation after 7 days when mixed with Puramatrix™ (peptide hydrogel), or after 14 days when mixed with recombinant human Collagen (rhCollagen) type I, and injected into the root canals of human premolars in vitro. Roots of human premolars injected with scaffolds (Puramatrix™ or rhCollagen) containing SHED were implanted subcutaneously into immunodeficient mice (CB-17 SCID). We observed pulp-like tissues with odontoblasts capable of generating new tubular dentin throughout the root canals. Notably, the pulp tissue engineered with SHED injected with either Puramatrix™ or rhCollagen type I presented similar cellularity and vascularization when compared with control human dental pulps. Analysis of these data, collectively, demonstrates that SHED injected into full-length human root canals differentiate into functional odontoblasts, and suggests that such a strategy might facilitate the completion of root formation in necrotic immature permanent teeth.
Keywords: biocompatible materials; dentinogenesis; neovascularization; odontoblasts; stem cells; tissue scaffolds.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures


References
-
- Casagrande L, Demarco FF, Zhang Z, Araujo FB, Shi S, Nör JE. (2010). Dentin-derived BMP-2 and odontoblastic differentiation of SHED. J Dent Res 89:603-608. - PubMed
-
- Chadipiralla K, Yochim JM, Bahuleyan B, Huang CY, Garcia-Godoy F, Murray PE, et al. (2010). Osteogenic differentiation of stem cells derived from human periodontal ligaments and pulp of human exfoliated deciduous teeth. Cell Tissue Res 340:323-333. - PubMed
-
- Cordeiro MM, Dong Z, Kaneko T, Zhang Z, Miyazawa M, Shi S, et al. (2008). Dental pulp tissue engineering with stem cells from exfoliated deciduous teeth. J Endod 34:962-969. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

