In vivo reprogramming of astrocytes to neuroblasts in the adult brain
- PMID: 24056302
- PMCID: PMC3867822
- DOI: 10.1038/ncb2843
In vivo reprogramming of astrocytes to neuroblasts in the adult brain
Abstract
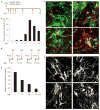
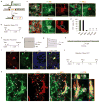
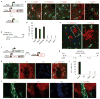
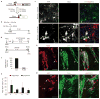
Adult differentiated cells can be reprogrammed into pluripotent stem cells or lineage-restricted proliferating precursors in culture; however, this has not been demonstrated in vivo. Here, we show that the single transcription factor SOX2 is sufficient to reprogram resident astrocytes into proliferative neuroblasts in the adult mouse brain. These induced adult neuroblasts (iANBs) persist for months and can be generated even in aged brains. When supplied with BDNF and noggin or when the mice are treated with a histone deacetylase inhibitor, iANBs develop into electrophysiologically mature neurons, which functionally integrate into the local neural network. Our results demonstrate that adult astrocytes exhibit remarkable plasticity in vivo, a feature that might have important implications in regeneration of the central nervous system using endogenous patient-specific glial cells.
Conflict of interest statement
The authors declare no competing financial interests.
Figures




Comment in
-
Glia: Reprogramming the residents.Nat Rev Neurosci. 2013 Nov;14(11):739. doi: 10.1038/nrn3620. Epub 2013 Oct 9. Nat Rev Neurosci. 2013. PMID: 24105344 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

