Mesenchyme-specific knockout of ESET histone methyltransferase causes ectopic hypertrophy and terminal differentiation of articular chondrocytes
- PMID: 24056368
- PMCID: PMC3820852
- DOI: 10.1074/jbc.M113.473827
Mesenchyme-specific knockout of ESET histone methyltransferase causes ectopic hypertrophy and terminal differentiation of articular chondrocytes
Abstract
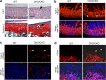
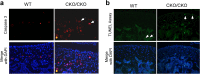
The exact molecular mechanisms governing articular chondrocytes remain unknown in skeletal biology. In this study, we have found that ESET (an ERG-associated protein with a SET domain, also called SETDB1) histone methyltransferase is expressed in articular cartilage. To test whether ESET regulates articular chondrocytes, we carried out mesenchyme-specific deletion of the ESET gene in mice. ESET knock-out did not affect generation of articular chondrocytes during embryonic development. Two weeks after birth, there was minimal qualitative difference at the knee joints between wild-type and ESET knock-out animals. At 1 month, ectopic hypertrophy, proliferation, and apoptosis of articular chondrocytes were seen in the articular cartilage of ESET-null animals. At 3 months, additional signs of terminal differentiation such as increased alkaline phosphatase activity and an elevated level of matrix metalloproteinase (MMP)-13 were found in ESET-null cartilage. Staining for type II collagen and proteoglycan revealed that cartilage degeneration became progressively worse from 2 weeks to 12 months at the knee joints of ESET knock-out mutants. Analysis of over 14 pairs of age- and sex-matched wild-type and knock-out mice indicated that the articular chondrocyte phenotype in ESET-null mutants is 100% penetrant. Our results demonstrate that expression of ESET plays an essential role in the maintenance of articular cartilage by preventing articular chondrocytes from terminal differentiation and may have implications in joint diseases such as osteoarthritis.
Keywords: Arthritis; Articular cartilage; Cartilage biology; Chondrocytes; Differentiation; Epigenetics; Gene Knockout; Histone methylation.
Figures




Similar articles
-
Premature induction of hypertrophy during in vitro chondrogenesis of human mesenchymal stem cells correlates with calcification and vascular invasion after ectopic transplantation in SCID mice.Arthritis Rheum. 2006 Oct;54(10):3254-66. doi: 10.1002/art.22136. Arthritis Rheum. 2006. PMID: 17009260
-
Generation and characterization of mice with mesenchyme-specific deletion of the entire ESET histone methyltransferase protein.Genesis. 2018 Feb;56(2). doi: 10.1002/dvg.23088. Epub 2018 Jan 13. Genesis. 2018. PMID: 29282851
-
ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates.Dev Biol. 2013 Aug 1;380(1):99-110. doi: 10.1016/j.ydbio.2013.04.031. Epub 2013 May 4. Dev Biol. 2013. PMID: 23652029 Free PMC article.
-
Articular cartilage and changes in arthritis. An introduction: cell biology of osteoarthritis.Arthritis Res. 2001;3(2):107-13. doi: 10.1186/ar148. Epub 2001 Jan 22. Arthritis Res. 2001. PMID: 11178118 Free PMC article. Review.
-
Cellular and molecular mechanisms of synovial joint and articular cartilage formation.Ann N Y Acad Sci. 2006 Apr;1068:74-86. doi: 10.1196/annals.1346.010. Ann N Y Acad Sci. 2006. PMID: 16831907 Free PMC article. Review.
Cited by
-
Histone Modifications and Chondrocyte Fate: Regulation and Therapeutic Implications.Front Cell Dev Biol. 2021 Apr 16;9:626708. doi: 10.3389/fcell.2021.626708. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33937229 Free PMC article. Review.
-
Mesenchyme-specific loss of Dot1L histone methyltransferase leads to skeletal dysplasia phenotype in mice.Bone. 2021 Jan;142:115677. doi: 10.1016/j.bone.2020.115677. Epub 2020 Oct 3. Bone. 2021. PMID: 33022452 Free PMC article.
-
Epigenetic regulation in chondrocyte phenotype maintenance for cell-based cartilage repair.Am J Transl Res. 2015 Nov 15;7(11):2127-40. eCollection 2015. Am J Transl Res. 2015. PMID: 26807163 Free PMC article. Review.
-
SETDB1-Mediated Silencing of Retroelements.Viruses. 2020 May 30;12(6):596. doi: 10.3390/v12060596. Viruses. 2020. PMID: 32486217 Free PMC article. Review.
-
Significance of epigenetic landscape in cartilage regeneration from the cartilage development and pathology perspective.Stem Cells Dev. 2014 Jun 1;23(11):1178-94. doi: 10.1089/scd.2014.0002. Epub 2014 Apr 1. Stem Cells Dev. 2014. PMID: 24555773 Free PMC article. Review.
References
-
- van der Kraan P. M., van den Berg W. B. (2012) Chondrocyte hypertrophy and osteoarthritis: role in initiation and progression of cartilage degeneration? Osteoarthritis Cartilage 20, 223–232 - PubMed
-
- Pitsillides A. A., Beier F. (2011) Cartilage biology in osteoarthritis–lessons from developmental biology. Nat. Rev. Rheumatol. 7, 654–663 - PubMed
-
- Berenbaum F. (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21, 16–21 - PubMed
-
- Yang L., Lawson K. A., Teteak C. J., Zou J., Hacquebord J., Patterson D., Ghatan A. C., Mei Q., Zielinska-Kwiatkowska A., Bain S. D., Fernandes R. J., Chansky H. A. (2013) ESET histone methyltransferase is essential to hypertrophic differentiation of growth plate chondrocytes and formation of epiphyseal plates. Dev. Biol. 380, 99–110 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

