Respiratory syncytial virus infection disrupts monolayer integrity and function in cystic fibrosis airway cells
- PMID: 24056672
- PMCID: PMC3798900
- DOI: 10.3390/v5092260
Respiratory syncytial virus infection disrupts monolayer integrity and function in cystic fibrosis airway cells
Abstract
Background: Respiratory Syncytial Virus (RSV) infection is a common contributor to pulmonary symptoms in children with cystic fibrosis (CF). Here we examined RSV infection in immortalized bronchial epithelial cells (CFBE41o-) expressing wild-type (wt) or F508del cystic fibrosis transmembrane conductance regulator (CFTR), for monolayer integrity and RSV replication.
Methods: CFBE41o- monolayers expressing wt or F508del CFTR were grown on permeable supports and inoculated with RSV A2 strain. Control experiments utilized UV-inactivated RSV and heat-killed RSV. Monolayer resistance and RSV production was monitored for up to six days post-infection.
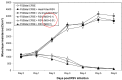
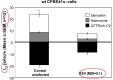
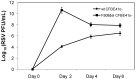
Results: Within 24 h, a progressive decrease in monolayer resistance was observed in RSV infected F508del CFBE41o- cells, while the monolayer integrity of RSV infected wt CFTR CFBE41o- cells remained stable. RSV replication was necessary to disrupt F508del CFBE41o- monolayers as UV-irradiated and heat killed RSV had no effect on monolayer integrity, with an earlier and much more pronounced peak in RSV titer noted in F508del relative to wt CFTR-expressing cells. RSV infection of wt CFBE41o- monolayers also resulted in blunting of CFTR response.
Conclusions: These findings identify an enhanced sensitivity of CFBE41o- cells expressing F508del CFTR to RSV infection, replication and monolayer disruption independent of the cellular immune response, and provide a novel mechanism by which cystic fibrosis airway epithelia are susceptible to RSV-dependent injury.
Figures

References
-
- Ward C.L., Kopito R.R. Intracellular turnover of cystic fibrosis transmembrane conductance regulator. Inefficient processing and rapid degradation of wild-type and mutant proteins. J. Biol. Chem. 1994;269:25710–25718. - PubMed
-
- Meyerholz D.K., Stoltz D.A., Namati E., Ramachandran S., Pezzulo A.A., Smith A.R., Rector M.V., Suter M.J., Kao S., McLennan G., et al. Loss of cystic fibrosis transmembrane conductance regulator function produces abnormalities in tracheal development in neonatal pigs and young children. Am. J. Respir. Crit. Care Med. 2010;182:1251–1261. doi: 10.1164/rccm.201004-0643OC. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

