Investigation of a regulatory agency enquiry into potential porcine circovirus type 1 contamination of the human rotavirus vaccine, Rotarix: approach and outcome
- PMID: 24056737
- PMCID: PMC3981850
- DOI: 10.4161/hv.25973
Investigation of a regulatory agency enquiry into potential porcine circovirus type 1 contamination of the human rotavirus vaccine, Rotarix: approach and outcome
Abstract
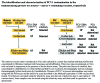
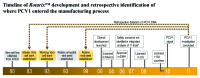
In January 2010, porcine circovirus type 1 (PCV1) DNA was unexpectedly detected in the oral live-attenuated human rotavirus vaccine, Rotarix (GlaxoSmithKline [GSK] Vaccines) by an academic research team investigating a novel, highly sensitive analysis not routinely used for adventitious agent screening. GSK rapidly initiated an investigation to confirm the source, nature and amount of PCV1 in the vaccine manufacturing process and to assess potential clinical implications of this finding. The investigation also considered the manufacturer's inactivated poliovirus (IPV)-containing vaccines, since poliovirus vaccine strains are propagated using the same cell line as the rotavirus vaccine strain. Results confirmed the presence of PCV1 DNA and low levels of PCV1 viral particles at all stages of the Rotarix manufacturing process. PCV type 2 DNA was not detected at any stage. When tested in human cell lines, productive PCV1 infection was not observed. There was no immunological or clinical evidence of PCV1 infection in infants who had received Rotarix in clinical trials. PCV1 DNA was not detected in the IPV-containing vaccine manufacturing process beyond the purification stage. Retrospective testing confirmed the presence of PCV1 DNA in Rotarix since the initial stages of its development and in vaccine lots used in clinical studies conducted pre- and post-licensure. The acceptable safety profile observed in clinical trials of Rotarix therefore reflects exposure to PCV1 DNA. The investigation into the presence of PCV1 in Rotarix could serve as a model for risk assessment in the event of new technologies identifying adventitious agents in the manufacturing of other vaccines and biological products.
Keywords: Rotarix™; adventitious agents; human rotavirus vaccine; inactivated poliovirus vaccine; porcine circovirus type 1.
Figures

References
-
- Office of the Federal Register. Code of Federal Regulations, 9 CFR and 21 CFR part 630 (Additional Standards for Viral Vaccines). 1996 and earlier versions. Available at: http://www.gpo.gov/fdsys/pkg/CFR-2001-title9-vol1/pdf/CFR-2001-title9-vo... Accessed July 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
