CSF-1R inhibition alters macrophage polarization and blocks glioma progression
- PMID: 24056773
- PMCID: PMC3840724
- DOI: 10.1038/nm.3337
CSF-1R inhibition alters macrophage polarization and blocks glioma progression
Abstract
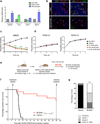
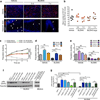
Glioblastoma multiforme (GBM) comprises several molecular subtypes, including proneural GBM. Most therapeutic approaches targeting glioma cells have failed. An alternative strategy is to target cells in the glioma microenvironment, such as tumor-associated macrophages and microglia (TAMs). Macrophages depend on colony stimulating factor-1 (CSF-1) for differentiation and survival. We used an inhibitor of the CSF-1 receptor (CSF-1R) to target TAMs in a mouse proneural GBM model, which significantly increased survival and regressed established tumors. CSF-1R blockade additionally slowed intracranial growth of patient-derived glioma xenografts. Surprisingly, TAMs were not depleted in treated mice. Instead, glioma-secreted factors, including granulocyte-macrophage CSF (GM-CSF) and interferon-γ (IFN-γ), facilitated TAM survival in the context of CSF-1R inhibition. Expression of alternatively activated M2 markers decreased in surviving TAMs, which is consistent with impaired tumor-promoting functions. These gene signatures were associated with enhanced survival in patients with proneural GBM. Our results identify TAMs as a promising therapeutic target for proneural gliomas and establish the translational potential of CSF-1R inhibition for GBM.
Figures




Comment in
-
Therapeutically reeducating macrophages to treat GBM.Nat Med. 2013 Oct;19(10):1207-8. doi: 10.1038/nm.3355. Nat Med. 2013. PMID: 24100977
-
Tumour microenvironment: Teaching old macrophages new tricks.Nat Rev Cancer. 2013 Nov;13(11):753. doi: 10.1038/nrc3616. Epub 2013 Oct 10. Nat Rev Cancer. 2013. PMID: 24108096 No abstract available.
References
-
- Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. - PubMed
-
- Balkwill FR, Mantovani A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin Cancer Biol. 2012;22:33–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

