Response endpoints and failure-free survival after initial treatment for acute graft-versus-host disease
- PMID: 24056814
- PMCID: PMC3912972
- DOI: 10.3324/haematol.2013.093062
Response endpoints and failure-free survival after initial treatment for acute graft-versus-host disease
Abstract
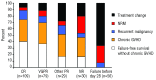
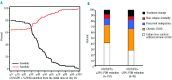
We evaluated short-term response endpoints for acute graft-versus-host disease treatment trials. We postulated that response endpoints should correlate with reduced symptom burden and decreased subsequent treatment failure, defined as non-relapse mortality, recurrent malignancy, or additional systemic treatment. The cohort included 303 consecutive patients who received initial systemic steroid treatment for acute graft-versus-host disease. Response was evaluated at day 28 after initial treatment, which in all cases preceded the onset of chronic graft-versus-host disease. At day 28, 36% of patients had a complete response, 26% had a very good partial response, 10% had another degree of partial response (other partial response) and 28% had no response. As expected, the symptom burden was lower in patients with very good partial response compared to those with other partial response. The frequencies of subsequent treatment failure were similar in patients with complete and very good partial responses, but lower than in patients with other partial response or no response at day 28. The frequency of second-line treatment was lower in patients with very good partial response than in those with other partial response. Risk factors associated with a lower probability of complete or very good partial response at day 28 were unrelated or human leukocyte antigen-mismatched related donor grafts and liver or gastrointestinal involvement at onset of initial treatment. Taken together, these results suggest that endpoints in acute graft-versus-host disease treatment trials should distinguish between very good partial response and other partial response. Our results support the use of complete or very good partial response at day 28 as an appropriate short-term primary endpoint.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

