Plasmonic nanoprobes: from chemical sensing to medical diagnostics and therapy
- PMID: 24056945
- PMCID: PMC4355622
- DOI: 10.1039/c3nr03633b
Plasmonic nanoprobes: from chemical sensing to medical diagnostics and therapy
Abstract
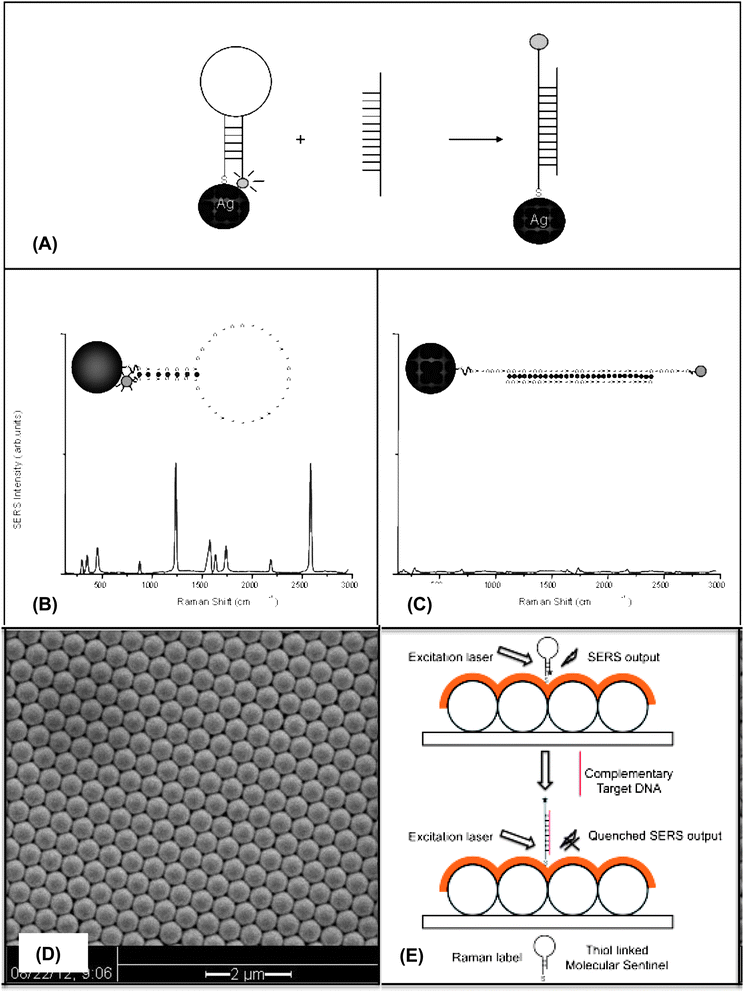
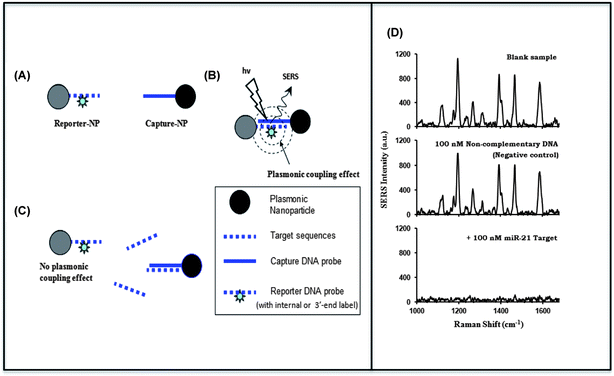
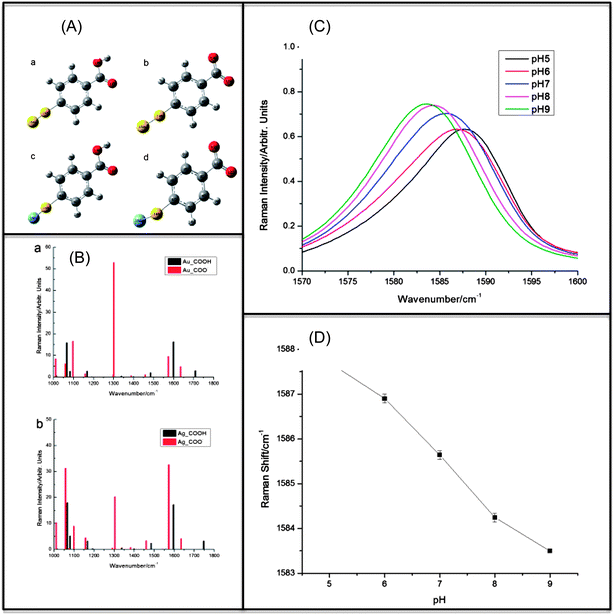
This article provides an overview of the development and applications of plasmonics-active nanoprobes in our laboratory for chemical sensing, medical diagnostics and therapy. Molecular Sentinel nanoprobes provide a unique tool for DNA/RNA biomarker detection both in a homogeneous solution or on a chip platform for medical diagnostics. The possibility of combining spectral selectivity and high sensitivity of the surface-enhanced Raman scattering (SERS) process with the inherent molecular specificity of nanoprobes provides an important multiplex diagnostic modality. Gold nanostars can provide an excellent multi-modality platform, combining two-photon luminescence with photothermal therapy as well as Raman imaging with photodynamic therapy. Several examples of optical detection using SERS and photonics-based treatments are presented to illustrate the usefulness and potential of the plasmonic nanoprobes for theranostics, which seamlessly combines diagnostics and therapy.
Figures






References
-
- Jeanmaire DL, Van Duyne RP. J. Electroanal. Chem. 1977;84:1–20.
-
- Albrecht MG, Creighton JA. J. Am. Chem. Soc. 1977;99:5215–5217.
-
- Moskovits M. Rev. Mod. Phys. 1985;57:783–826.
-
- Wokaun A, Gordon JP, Liao PF. Phys. Rev. Lett. 1982;48:957–960.
-
- Schatz GC. Acc. Chem. Res. 1984;17:370–376.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

